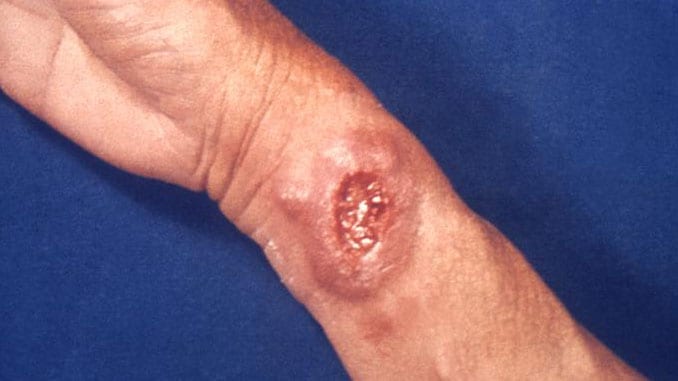
The U.S. Army Medical Material Development Activity (USAMMDA) is conducting market research to survey available or emerging technologies for a diagnostic device to rapidly diagnose cutaneous leishmaniasis. Ideally the device would be used in point-of-care settings.
Manufacturing of the device would need to comply with cGMP requirements as FDA clearance would be ultimately required.
Candidate devices should be at the Technology Readiness Level 5 (TRL 5). If the device development is at the proof of concept stage (TRL 3) and current manufacturing is not cGMP, companies should include a gap analysis and estimated time by which cGMP manufacturing could be achieved.
Additional requirements on sensitivity, specificity and shelf-life are outlined in the formal announcement here. The response deadline is 12 January, 2012.