 The National Institute of Allergy and Infectious Diseases (NIAID) has made multiple funding awards in support of Vaccine and Treatment Evaluation Units (VTEU).
The National Institute of Allergy and Infectious Diseases (NIAID) has made multiple funding awards in support of Vaccine and Treatment Evaluation Units (VTEU).
The VTEUs, supported by the NIAID since the 1960’s, have designed and conducted a broad range of clinical studies and clinical trials of bacterial, viral and parasitic vaccines, therapeutics, and other biologics and drugs as preventive and therapeutic measures against infectious diseases in people of all ages and risk categories.
The award amounts for each institution are a minimum value of $100,000 and maximum of $951,702,000. The recipients are:
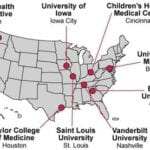
- Baylor College of Medicine
- Children’s Hospital Medical Center
- Duke University
- Emory University
- Group Health Research Institute
- University of Iowa
- Saint Louis University
- University of Maryland, Baltimore
- Vanderbilt University Medical Center
The VTEU contractors must be able to perform identification, design, development, implementation, analysis and reporting of clinical studies and clinical trials. Tasks and support functions executed by the organizations improve the understanding, diagnosing, treating, and preventing infectious diseases caused by viral, bacterial, parasitic and fungal pathogens, including the National Institute of Allergy and Infectious Diseases (NIAID) Priority Biodefense Pathogens and emerging infectious disease pathogens.
The awards were announced under Solicitation Number: RFP-NIAID-DMID-NIHAI2012144.


