Bacteria can evolve rapidly to adapt to environmental change. When the “environment” is the immune response of an infected host, this evolution can turn harmless bacteria into life-threatening pathogens. A study published on this week in PLOS Pathogens provides insight into how this happens.
Isabel Gordo and colleagues from the Instituto Gulbenkian de Ciencia in Oeira, Portugal, have for the first time devised an experimental system to observe and study the evolution of bacteria in response to encounters with cells of the mammalian immune system. They found that in less than 500 bacterial generations (or 30 days), the bacteria became more resistant to being killed by immune cells and acquired the ability to cause disease in mice.
“Escherichia coli bacteria show an extraordinary amount of diversity: Many are benign commensal bacteria, but some are deadly pathogens”, says Isabel Gordo. “It is thought that many strains of E. coli that cause disease in humans evolved from commensal strains. We thought that experimental evolution would be a powerful tool to directly observe some of the steps E. coli may take in the transition from commensalism to pathogenesis.”
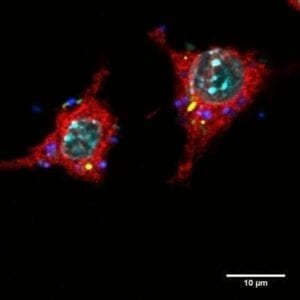
For their study, the scientists studied initially benign E. coli bacteria that were continuously confronted with macrophages, which are part of our immune system and can swallow and digest bacteria. They grew a mix of bacteria and macrophages in a liquid culture (a glass bottle that contains a nutritious broth). Once a day, they diluted the mix, and every other day they took a sample of the bacteria for further analysis. As a control, they grew, diluted, and analyzed bacteria from the same ancestral strain but grown without macrophages.
From day four on, bacteria that had been exposed to macrophages started to show changes in their phenotype (their appearance), whereas such changes were never observed in the controls. The selective pressure imposed by the presence of the macrophages prompted changes in the bacteria that were consistently observed in six independent experimental series. The changes affected the phenotype of the bacteria (with new variants forming either “small colonies” or “mucoid colonies”), their fitness, and their genetic make-up.
When the scientists looked at the interaction between new variant bacteria and macrophages more closely, they found that the small colony variants were more resistant to being digested by macrophages than the ancestral strain, and the mucoid variant was less likely to be gobbled up. When they infected mice with mucoid variant bacteria, they also found that the variants have increased ability to cause disease in mice.
“We demonstrate”, the scientists say, “that E. coli can adapt to better resist macrophages within a few hundred generations, and that clones with morphologies and traits similar to those of pathogenic bacteria rapidly emerge”.
Read the study at PLOS Pathogens: The Genetic Basis of Escherichia coli Pathoadaptation to Macrophages.


