Every year the approach of flu season sets off a medical guessing game with life or death consequences. There are many different strains of flu and they vary from year to year. So each season authorities must make an educated guess and tell manufacturers which variants of the flu they should produce vaccines against.
Even when this system works, flu-related illnesses can kill 3,000 to 49,000 Americans annually, according to the Centers for Disease Control and Prevention. A bad guess or the unexpected emergence of a virulent strain could send the death toll higher.
Against this backdrop Stanford researchers report promising steps toward the creation of a universal flu vaccine, one that could be produced more quickly and offer broader protection than the virus-specific inoculants available today.
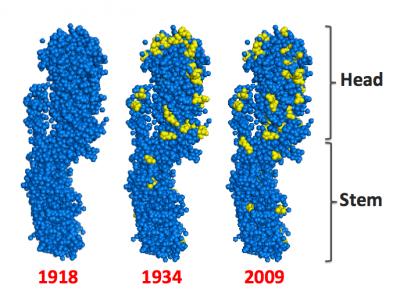
This image shows the HA protein from three flu strains. The HA protein is the target for current vaccines. This protein has a head and a stem. Current vaccines target the head. The blue and yellow dots depict the amino acids that make up the HA protein. The yellow dots show amino acids that change in the 1934 and 2009 protein, relative to the 1918 variant. There are more changes in the head than in the stem. Stanford researchers are developing a flu vaccine based on the stem of the HA protein. If successful, this could yield a vaccine that protects against different strains of flu, and perhaps for more than one year at a time. Credit: Yuan Lu, Stanford University
The researchers detail their work in the current edition of the Proceedings of the National Academy of Sciences. The team was led by chemical and bioengineer James R. Swartz, who is the James H. Clark Professor in the School of Engineering.
Their approach arises from a better understanding of the structure of a key protein on the surface of the flu virus, and a new process for making vaccines based on that understanding.
A flu virus is made up of different proteins. Protruding from the surface of the virus are hundreds of copies of a protein called Hemagglutinin (HA). Each copy of HA resembles a mushroom, with a head and a stem. The head of HA helps determine the virulence of a given strain of flu.
Today’s vaccines are based on inactivated viruses that contain the heads of HA proteins. When a flu shot is injected into our blood stream, our immune system sees the HA head as a target, and creates antibodies to fight what appears to be an infection.
Teaching the immune system to recognize a target is the essence of vaccination. If we are exposed to the flu after getting vaccinated, our immune system is primed to recognize and eradicate the invading virus before it can replicate sufficient copies to make us sick.
Swartz and his colleagues base their new vaccine approach on the understanding that, whereas the head of the flu virus varies from year to year, the protein stem remains more constant over time.
Theoretically, a vaccine based on the stem should be more broadly protective against different strains of flu, and perhaps offer universal protection. Moreover, since the stem remains relatively constant from year to year, once our immune systems produces antibodies against that antigen, multi-season protection might be possible.
But this approach remains experimental and has not yet been tested on patients.
The Stanford paper focused on the first step in developing such a universal vaccine: creating a protein stem fragment that could be injected into the blood stream, in short, creating a target, or antigen, to attract the attention of our immune system and trigger an effective defense.
Yuan Lu, a postdoctoral scholar in Swartz’s lab and member of the research team, outlined the process detailed in the PNAS paper. The researchers began with a section of DNA that contained the instructions for making the protein structure for one important strain of flu, the H1N1 virus that caused the pandemic of 1918 and recurred in milder form in 2009.
The researchers started with the DNA sequence that defines the entire HA protein, both head and stem. They then subtracted the DNA coding for the head. Thus, their edited DNA strand only contained the instructions for making the protein stem.
The Stanford team used a relatively new and experimental process to manufacture the viral stem. This process is called cell free protein synthesis (CFPS).
Inside all cells there are molecular machines called RNA polymerases and ribosomes. These RNA polymerases and ribosomes “read” DNA to manufacture proteins based on the instructions in the genetic code.
In cell free protein synthesis, scientists bust open bacterial cells to create a molecular goop that contains a lot of these ribosomes. Scientists know how to transmit their DNA instructions directly to these protein factories.
The advantage of CFPS is that it can produce proteins in a few hours versus a couple of weeks or even a couple of months, which is how long it takes to make proteins for flu vaccines using the practices that are approved for medical use today.
The Stanford researchers used this CFPS process to create and refine a viral protein stem that would be useful as an experimental vaccine antigen.
To do this they had to solve two fundamental problems. First, the CFPS process produced a single-strand protein, or monomer. But the HA stem is a trimer, or three identical monomers braided together. Second, their bioengineered antigen was not initially soluble. In other words, it could not be made into a liquid vaccine form.
Remember that everything started with a DNA sequence. Making the antigen involved feeding a DNA sequence into the molecular goop containing RNA polymerases and ribosomes, extracting the viral protein stems, and determining whether they had created soluble trimers that had at least the potential to be injectable antigens.
The researchers went though dozens of experiments to produce monomers that could fold into soluble trimers. Proteins are made of smaller building blocks called amino acids. Changing the structure of the protein stem therefore involved editing the DNA to change specific amino acids, running these new instructions back through the ribosome factories, extracting the finished product and testing the results.
It took dozens of tries over two years but eventually the researchers fed a DNA snippet into the CFPS process and created a soluble viral stem protein that could be a good antigen. That is what they report in the PNAS paper. “This has been a tough process,” Swartz said. “Many labs have been trying to develop an HA stem vaccine and we’re glad to have made these contributions.”
Many steps remain before the research community knows whether this viral stem approach yields a better flu vaccine. Next, Swartz and his team will attach their stem protein to a virus-like particle. The idea will be to create a bigger, better target with which to elicit an immune system response.
Should that prove successful, the new vaccine candidate would have to undergo safety and efficacy tests in animals, and eventually, large scale human clinical trials. Much is at stake. Recent estimates put the worldwide death toll from flu-related illnesses at between 250,000 and 500,000 persons per year.
“This is an important project for world health,” Swartz said, noting that the vaccine must not only be broadly effective against different strains of flu but cheap to produce so that it can be widely distributed. “These are big challenges but we are committed to the effort.”
School: Stanford School of Engineering