A team of biologists from San Diego State University has developed a platform for identifying drugs that could prove to be effective against a variety of viral diseases.
In a pair of recent articles in the Journal of Biomolecular Screening and the Journal of Visualized Experiments, the researchers describe how the methodology works, using dengue virus as an example, and they identify a novel drug which may someday be used to combat the disease.
Over the past several years, the researchers, led by SDSU biologist Roland Wolkowicz, have been developing cell-based platforms that can be used to monitor the biomolecular activity of viruses inside their host cells. Based on a platform previously created for HIV, Wolkowicz, together with his team, built a new platform for dengue virus.
According to data from the World Health Organization, dengue infects between 50 and 100 million people each year and has no known vaccine or treatment. Some 22,000 people die each year from the disease.
Critical Cleavage
Like all viruses, dengue has no independent means of replicating itself. It hijacks the cellular machinery of its host to copy its genetic material and spread new viral particles. In order to do this successfully, the virus relies on a number of complex protein functions.
The Wolkowicz team’s platform screens host cells to detect whether or not a particular protein function of dengue, known as pre-membrane protein (prM) cleavage, has occurred. The prM cleavage process is important for new viral particles to be able to infect a host cell. Preventing the cleavage from happening could effectively stop the virus in its tracks.
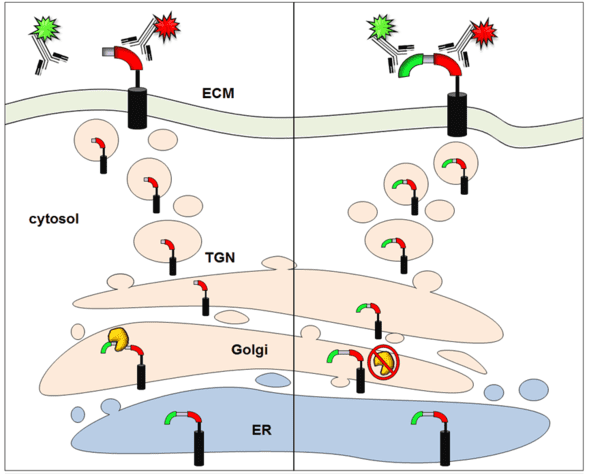
“Cleavage is absolutely critical to the virus’s life cycle,” explained Wolkowicz, corresponding author on the Journal of Biomolecular Screening paper. “While the role of prM cleavage is not completely understood, new particles won’t mature without it. By blocking prM cleavage, you clearly diminish the virus’s ability to infect other cells.”
Tagging Up
His lab’s technique embeds two biomolecular tags — a “red” tag and a “green” tag, so-called because of the fluorescent antibodies that detect them — inside an infected cell. These tags surround a portion of the virus’s prM protein and go along for the ride when it is transported to the cellular surface. The tags are designed in such a way that if cleavage occurs, one of them falls off.
“If you get cleavage, you’ll lose the green tag,” said Cameron Smurthwaite, manager of SDSU’s flow cytometry core facility and lead author on the Journal of Visualized Experiments paper.
As these tags make their way to the cell’s surface while prM cleavage occurs, fluorescing antibodies let the researchers know whether the cleavage was successful or not. A solitary red tag means cleavage did occur and the virus is able to export new infectious viral particles. But if the researchers see the green tag, it means cleavage was blocked and the viral process was stopped.
Using this technique, Smurthwaite and Zach Stolp, formerly an SDSU student and currently a doctoral student at Johns Hopkins University, tested 1,280 drug compounds to see whether any of them could block prM cleavage to yield a green tag, preventing a critical step in the dengue viral life cycle.
“All of them but one were negative for green,” Smurthwaite said.
Robust Platform
The single successful drug was a compound known as Thiostrepton, which is known to have antibiotic properties but has never been connected with dengue virus. Wolkowicz stressed that this finding shouldn’t be interpreted as any kind of cure for the disease. It is possible that Thiostrepton might have some therapeutic effect against dengue, but verifying that will require a great deal more research and clinical testing.
Instead, Wolkowicz said his team’s results demonstrate just how robust and powerful their platform is for identifying drug candidates for fighting viruses. He added that it is relatively easy to adapt the platform to screen for the same protein pathway in other viruses such as HIV, West Nile, chikungunya and others. It’s even possible to screen drugs against multiple viruses at the same time.
Doing so could lead not only to new antiviral drugs, but also to a better understanding of viral biology in general.
“If the platform is robust, and ours is, you can imagine using it to screen thousands and thousands of compounds against different viruses for the discovery of new antivirals,” Wolkowicz said. “And it could be important for finding factors critical to the life cycle of viruses.”


