The U.S. Department of Health and Human Services (HHS) intends to hold a prize competition in which up to $20 million will be made available, subject to the availability of funds, for the delivery of one or more successful rapid point-of-care (POC) diagnostics.
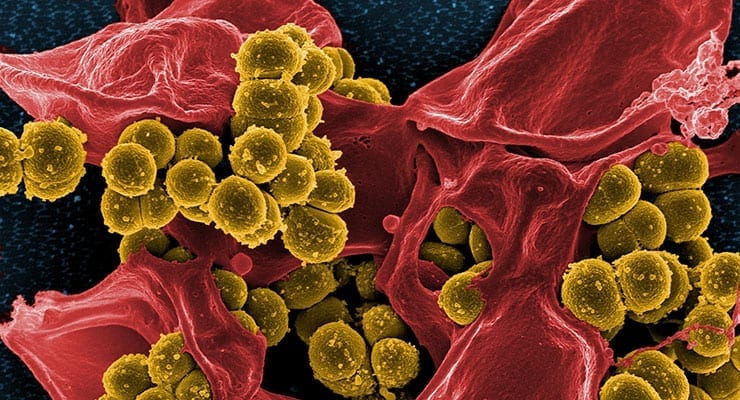
The goal of the program is advanced development of innovative diagnostic tests for identification and characterization of resistant bacteria for use by health care providers.
Such a diagnostic test could be used to help guide decisions about the necessity of prescribing antibiotics, and if so, which antibiotics may be effective – thus promoting antibiotic stewardship.
Another important diagnostic use could be to facilitate clinical trials for new antibacterial products by allowing for the enrichment of patient populations with specific infections, advancing the development of new antibacterial agents.
The National Institutes of Health (NIH) and the Biomedical Advanced Research and Development Authority (BARDA) are sponsoring the prize competition, and the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are contributing technical and regulatory expertise to develop the award evaluation process.
The current Request for Information (RFI) seeks public comments regarding the technical criteria and performance characteristics of the diagnostics for which the prizes will be offered.
Input received during this 45-day comment period and during the subsequent public consultation will be used by HHS to develop the technical criteria and performance characteristics of the diagnostics for which the prizes will be offered.
Further details are available via Notice Number: NOT-OD-15-104. The response deadline is July 17, 2015.