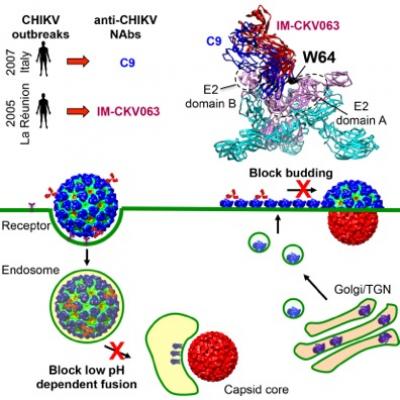
Scientists looking at the antiviral mechanisms of two previously identified human monoclonal antibodies have found they potently inhibit chikungunya virus (CHIKV) at multiple stages of infection.
Funded by grants from the National Institutes of Health, scientists at Blood Systems Research Institute (BSRI) in San Francisco and the Washington University School of Medicine in St. Louis have found that neutralizing antibodies that engage epitopes including residue E2-W64 are highly potent at inhibiting the virus in mice.
Furthermore, these antibodies prevent CHIKV from both entering and exiting cells, whereas prior studies of neutralizing antibodies to CHIKV and multiple other classes of viruses have focused on the capacity to block viruses from entering a cell.
Dr. Graham Simmons, associate investigator at BSRI and the lead researcher on the project, together with Dr. Jing Jin, a postdoctoral fellow in his laboratory, view these recent discoveries in the scope of a greater body of work that he and other researchers are doing to understand and combat CHIKV and other viruses.
“Inhibiting chikungunya virus, both at the points of entry and release from cells, is another important piece in the puzzle that could lead to new approaches in therapeutics and vaccines to fight infectious diseases,” explains Dr. Graham Simmons, associate investigator at BSRI and the lead researcher on the project.
Last month, a team led by Dr. Michael Diamond of the Washington University School of Medicine in St. Louis published related research which found that a panel of cross-neutralizing monoclonal antibodies can potently inhibit both entry and release of more than one alphavirus.
This result, effectively a method for blocking the spread of alphaviruses between cells, is one that could eventually lead to a single vaccine that protects against multiple viruses. Such a vaccine would represent a major milestone in global infectious disease prevention.
“But for chikungunya specifically,” continues Simmons, “more research is still needed to know whether the virus can be transmitted via blood transfusion and if the recipient of the infected blood develops symptoms.”
In the past decade, CHIKV, a mosquito-borne virus, has spread from endemic areas of Africa and Asia to Europe and the Americas, infecting millions. Infection causes high fever, often accompanied by severe joint pain, which can lead to chronic arthritis. It is not yet known if CHIKV is transmitted through blood transfusion from an infected donor; no cases of transfusion-transmitted CHIKV have been reported to date. Currently, there is no FDA-approved vaccine or blood screening test for CHIKV, and research continues.


