Elusys Therapeutics, Inc. announced today that the company has delivered the first doses of ANTHIM® (obiltoxaximab) Injection, its treatment for inhalational anthrax, to the U.S. Strategic National Stockpile (SNS), the U.S. Government’s repository of critical medical supplies for public health emergency preparedness.
The company is providing this delivery of ANTHIM as part of a procurement contract totaling $44.9M issued in 2015 by the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response (HHS ASPR).
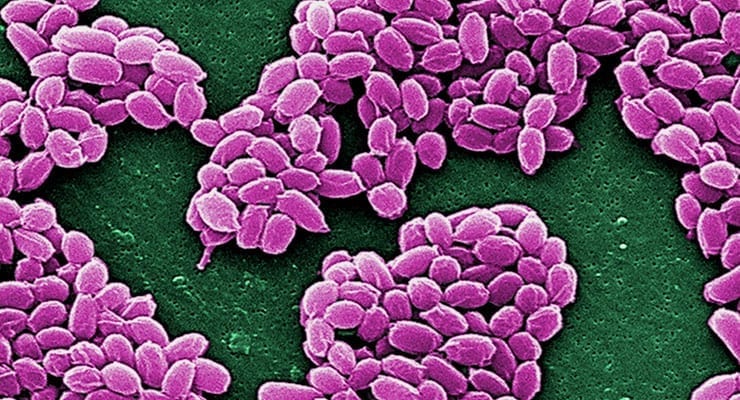
ANTHIM is a monoclonal antibody that binds to the protective antigen (PA) component of anthrax toxin. ANTHIM’s toxin neutralizing activity prevents entry of anthrax toxin into susceptible cells, avoiding further spread of the toxin throughout the body and the ensuing tissue damage that leads to death. ANTHIM is supplied as single-dose vials for IV infusion.
ANTHIM received U.S. Food and Drug Administration (FDA) marketing approval in March 2016. ANTHIM is indicated in adult and pediatric patients for the treatment of inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs, and for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate.
Elusys has received over $240M in grants and contracts from the U.S. Department of Defense (DoD), National Institutes of Health (NIH), and BARDA, and is now partnering with the U.S. Centers for Disease Control and Prevention (CDC), which manages the SNS, to ensure that this lifesaving countermeasure can be provided in the event of an anthrax outbreak.
Elusys also announced today that it continues to make progress in developing an alternate dosage form for ANTHIM with the initiation of a manufacturing campaign to produce material for completion of the lyophilization (freeze drying) commercial process, in addition to providing product to complete the 2015 procurement order.
The lyophilized form of ANTHIM is expected to extend product shelf-life by several years. Lyophilization also may improve tolerance of ANTHIM to extreme temperatures during shipping and field use.