The National Institute of Environmental Health Sciences (NIEHS), in support of the National Toxicology Program (NTP), is conducting market research to formulate a procurement strategy for obtaining support to examine a variable number of test articles annually for induction of genetic changes.
For this requirement, the contractor must have the capability to:
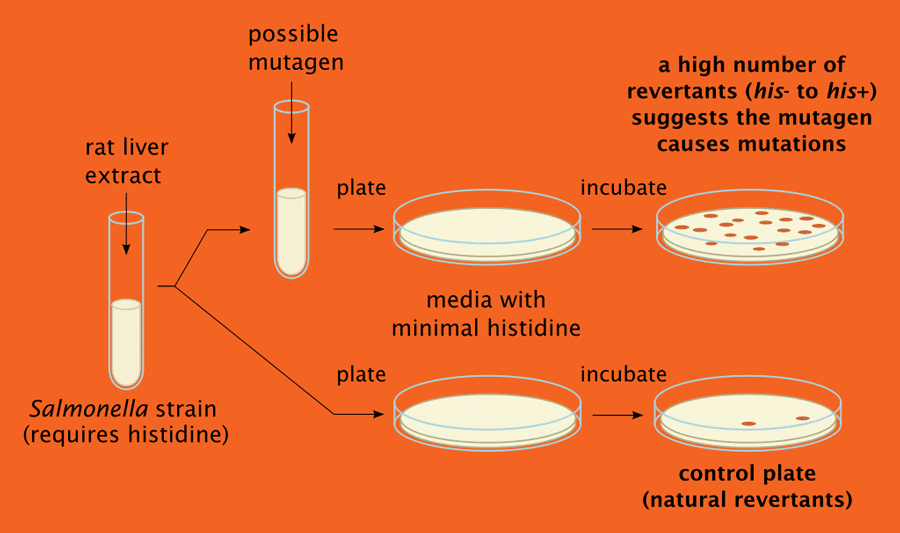
- Test approximately 20 chemicals per year for induction of gene mutations in bacterial tester strains (Ames test)
- Measure chromosomal changes and DNA damage in tissue samples obtained from laboratory animals and shipped to the contractor from a remote NTP testing laboratory
- Approximately 400 such samples may be evaluated annually
- Test approximately 20 chemicals per year for induction of chromosomal changes and DNA damage in human and mammalian cell cultures
- Measure molecular biomarkers of genetic change including but not limited to gene expression changes and DNA sequence changes.
The contractor should have experience conducting high throughput (96-well) in vitro mammalian cell assays for DNA damage and chromosome damage to enhance efficiency and statistical power. Data from in vivo and in vitro micronucleus assays will routinely be collected using flow cytometry methods.
Additional requirements are detailed in Solicitation Number: NIHES2019_RFI_GENETOX.