Microbiologist Ronald Corley has gone to work every day throughout the pandemic as director of the National Emerging Infectious Diseases Laboratories. Within this secure lab facility in Boston, scientists study pathogens as diverse as tuberculosis, Ebola virus, yellow fever virus and Zika virus. Many investigators there quickly turned their attention in 2020 to SARS-CoV-2, the virus that causes COVID-19.
Here Corley answers some of the most frequently asked questions about this kind of biosecure lab and the work researchers do inside it.
What is the purpose of a biocontainment facility?
A newly emerging or reemerging human pathogen is detected somewhere around the globe every 12 to 18 months.
Infectious diseases don’t respect borders. Because of the global economy and unprecedented mobility, everyone on the planet is vulnerable to potentially devastating infectious diseases that may have originated halfway across the world. In this age of high-speed travel, we are as little as 36 hours away from any outbreak.
As with SARS-CoV-2, scientists may know little about emerging pathogens or the diseases they cause. Studying these germs – whether bacteria, viruses or other microorganisms – in the safe environment of a biocontainment laboratory is the best protection humankind has against these diseases. In the lab, researchers can safely test new diagnostics, therapeutics and vaccines. The more scientists learn about these new diseases, the better prepared we are for the ones that will come after.
This is where labs like the NEIDL, and our stringent safety measures, are important. I feel safer from infection working in the NEIDL than I do in my apartment building. We know what we’re working with in the lab and how to keep ourselves and others safe. But outside, I don’t know who I might pass who could have a transmissible pathogen, including the coronavirus.
This is not to say that there is no risk working within the laboratory – there is. But we minimize it through a series of safety measures – including building systems, laboratory design, personal protective equipment, training and safety protocols – that have been tried and tested in laboratories across the world.
How do you try to minimize risk?
Our biosafety manual sets the standards for all work with biological material in the NEIDL. Requirements increase in complexity from Biosafety Level 2 (BSL-2) on to BSL-3 and BSL-4.
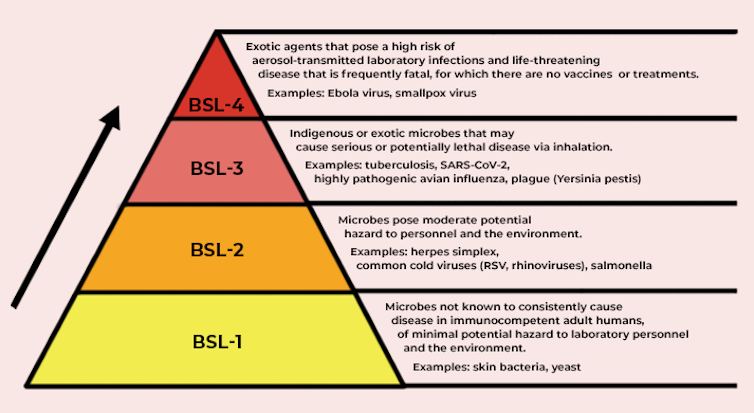
In the U.S., the Centers for Disease Control and Prevention determines each pathogen’s biocontainment level, based on what’s known about how it infects its host, the severity of the disease it causes, how easily transmissible the pathogen may be and the nature of the work itself – does it potentially create aerosols, for example.
The biosafety levels require different types of engineering controls – such as the building materials the space uses, directional air flow to ensure pathogens can’t get out, HEPA filtration so that only sterile air is discharged from the lab space and so on.
The administrative controls required vary by biosafety level, as well – safety protocols, requirements for personnel training, limiting access and so forth.
Each level requires different types of personal protective equipment: gloves and lab coats in a BSL-2 laboratory, protective lab wear and N95 or PAPR respirators in BSL-3 or a fully encapsulating suit in a BSL-4 laboratory.
“Safety First” is not just a bumper-sticker phrase at the NEIDL. Everyone from public safety officers to support staff to researchers has fully bought into the culture of safety. It informs the way we’re trained and drilled, the way pathogens are transported to the facility, and policies that govern our employees. We know the risks of the work, train on protective measures, and ensure every member of our staff follows our protocols.
What does containment look like with these safety strategies in place?
Everyone undergoes annual background checks, medical clearances and training. Only cleared staff can enter the building alone.
There are limited ways into the space, one for pedestrians, and one for vehicles, like delivery trucks. Entry requires access via biometric or card access or both, and screening by security. Access controls limit staff members to entering spaces where they have permission to work, based on their training, clearances and biosafety protocols. A network of security systems and closed-circuit cameras monitors the facility.
Entering laboratories requires that workers don the appropriate PPE for the area. Within the labs, we know what pathogen we are working with and how it is being used and are confident staff are following the safety measures required to keep them safe. This ensures the safety of others in the building as well as the surrounding community.
Importantly, the biosafety practices ensure that each pathogen we’re studying is restricted to the appropriate spaces. Researchers work at biosafety cabinets that sterile-filter the air before releasing it back into the lab.

What kinds of regulation and oversight are there?
Biocontainment laboratories do not function in a vacuum. The building and laboratory designs, and the PPE and operating procedures that protect staff, meet the guidelines set by the CDC and by the 574-page book “Biosafety in Microbiological and Biomedical Laboratories” from the CDC and National Institutes of Health.
To carry out a project, the lead scientist begins with an application to the Institutional Biosafety Committee. Experts in biosafety and science review the application, as do laypersons who provide a community perspective. These deliberations are open and transparent thanks to public participation on the committee. Its minutes are posted online. Safety professionals also inspect the laboratory facilities before work gets underway.
In the city of Boston, projects that involve any BSL-3 or BSL-4 work require review and approval from the Boston Public Health Commission, one of the only local public health departments with this type of oversight. Work with certain types of pathogens called “select agents” that pose a severe threat is further regulated by the Division of Select Agents and Toxins within the CDC.
Here at the NEIDL, both city and federal officials inspect the laboratories, interviewing personnel and reviewing records, including maintenance records. They also inspect pathogen inventories. Inspections can be announced or unannounced.
What would happen if something went wrong?
An important aspect of safety is making sure everyone knows what to do in an emergency. Three trainings per year involve first responders from the city as well as from Boston University. These are done as either live drills or tabletop exercises with experts walking through what an emergency would look like. Afterward we review how we did and develop plans for improvement.
Community members are also part of the exercises, and this keeps our neighbors involved and hopefully provides assurance of our ability to handle accidents, keeping ourselves and the community safe.
At Boston University, we post all laboratory incidents, including those at the NEIDL, on a quarterly basis to ensure that we remain transparent in our activities. Depending on what went wrong, we may also report to the BPHC and the CDC.

Why place these high-security labs in urban environments with lots of neighbors instead of the middle of nowhere?
Scientific research is a communal activity, and advances happen in places where diverse expertise is concentrated. It’s no different for research on emerging pathogens. Research on pathogens relies on faculty with expertise in not only the pathogens themselves but chemistry, engineering, stem cell biology, structural biology, immunology and more.
Biocontainment research also requires facilities engineers, safety professionals and security personnel. You can find personnel with diverse experience and expertise in metropolitan areas that are already home to biomedical research.
The original permitting process of the NEIDL mandated a comprehensive risk assessment to determine any potential danger for the community. After two years and independent review by two scientific panels, we ended up with the most extensive analysis of risk for any BSL-3 or BSL-4 facility in the U.S. It considered hundreds of possible scenarios that might result in exposure of a worker to a pathogen, or the release of a biological agent. The report concluded that it’s as safe, or even safer, to have such a facility in an urban environment than in a rural or suburban environment.
“Near misses” have occurred at these kinds of labs within the U.S. and Europe. A near miss might, for example, involve glove tears and a potential exposure to a pathogen during laboratory work, but these have never resulted in any community infections. At the NEIDL, we intend to maintain this track record.

What are the risks of not doing this research?
Science builds on what’s been learned before, accelerating our ability to respond to new outbreaks. The data we generate speeds progress on other pathogens as well, and informs how we develop and test potential therapeutics and vaccines. The risk of not doing this work is to leave ourselves more vulnerable to emerging pathogens as they arise.
Professionals working on emerging infectious diseases are interested in solving problems that benefit the public’s health. We take pride in our work and are serious about our responsibility to perform our work safely and securely. We recognize that this research is often viewed skeptically and thus strive to keep the trust of the public by ensuring transparency around the work we do.
Ronald Corley is the Director of the National Emerging Infectious Diseases Laboratories (NEIDL) and Chair of Microbiology, Boston University. Dr. Corley’s research focuses on the relationship between innate and adaptive immunity, and how components of the two discrete systems interact to generate long-lasting protective immune responses. One area of focus has been on the role of IgM antibodies as efficient bridges of innate and adaptive immunity. IgM antibodies serve to concentrate antigens into secondary lymphoid organs, accelerating the production of protective immune responses along with affinity matured antibodies. IgM also contributes to preventing pathogens from initially localizing into vital organs. The innate functions of IgM can be attributed, at least in part, to its ability to concentrate immune complexes in the foci of the spleen, where innate B cells in the marginal zones traffic the complexes to follicular dendritic cells, stromal elements that promote efficient germinal center formation. These functions require native organization around the marginal sinuses for efficient adjuvant effects of IgM. The laboratory also focuses on the immune consequences of infection with highly pathogenic hemorrhagic fever viruses, including filoviruses. Patients infected with these viruses often fail to make adaptive immune responses and succumb to infection. While this is often attributed to dysfunctional innate immune responses, the nature of the defects in immune responses to these viruses is poorly understood. Studies focus on the very early immune responses to these viruses, in order to map how they affect the ability of infected hosts to initiate adaptive immune responses, and on identifying the components of infection that lead to abnormal cytokine responses that characterize infection with these viruses.
This article is courtesy of The Conversation.



