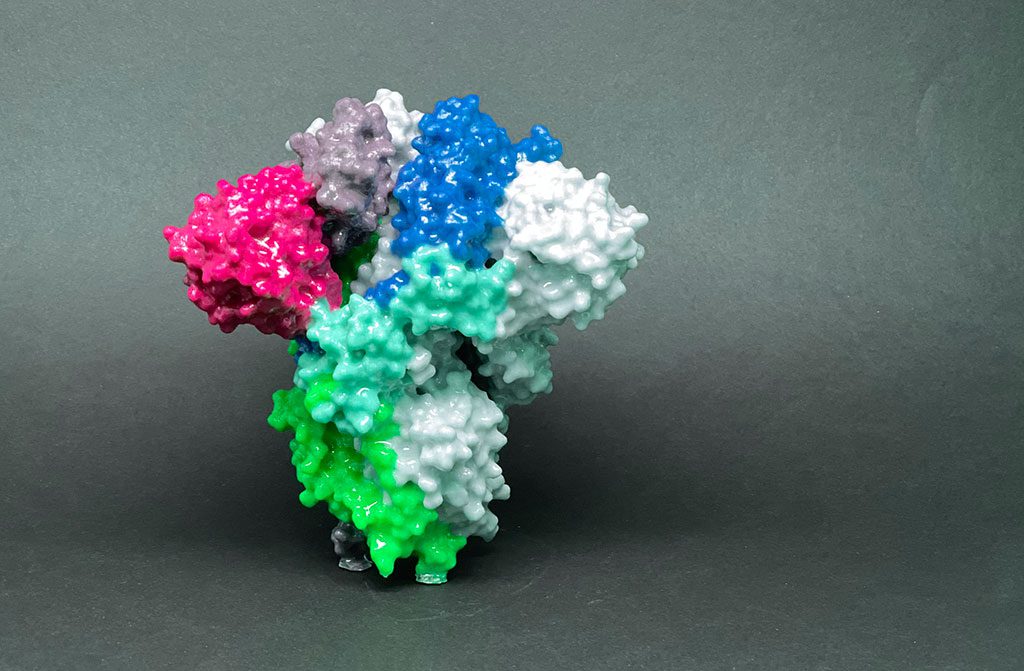Hundreds of antibodies contributed by over 50 different organizations around the world were classified and mapped. This study shows exactly where each antibody binds on the Spike protein of SARS-CoV-2.
Scientists at La Jolla Institute for Immunology (LJI) have published a detailed map of where human antibodies bind to SARS-CoV-2, a map that was generated by a global collaboration comparing nearly all leading clinical candidates.
“We were able to map the geography of Spike and understand which antibodies bind to which footprints. This map provides a reference to help predict which antibodies are still effective against SARS-CoV-2 variants of concern like the currently surging Delta variant,” says LJI Professor Erica Ollmann Saphire, Ph.D., who leads the global effort behind this research, called the Coronavirus Immunotherapeutic Consortium (CoVIC).
The researchers describe the neutralizing strength, or potency, of each antibody and the likelihood that each antibody could offer protection against viral variants. Antibodies with similar footprints on Spike were grouped into “communities.” The researchers show how antibodies from different communities could be combined in a powerful antibody “cocktail” to target the virus.
In fact, the researchers found three different groups of antibodies that are resistant to mutations in SARS-CoV-2 Spike protein. These antibodies could target vulnerable sites on the Spike protein, even as it mutates.
“We now have a framework for selecting durable antibody cocktails for COVID-19 treatment,” says Saphire.
Gathering Powerful Antibodies
The CoVIC includes about 370 antibodies from 59 different discovery efforts that cover a broad range: from academic labs and small biotechs to large international pharmaceutical corporations. These antibody therapeutics are being evaluated side-by-side in standardized lab by seven different partner labs. The Saphire team at LJI is determining high-resolution structures of these antibodies and also rapidly generated tools to examine the effects of mutations in Spike on antibody potency.
“CoVIC was formed to analyze a huge panel of monoclonal antibodies on equal footing,” says study co-first author Kathryn Hastie, Ph.D., an instructor at LJI. “The initial goal was to look at antibodies against the original SARS-CoV-2 strain, but it quickly became clear that the virus’s Spike protein was ever-evolving. The ability of the Spike to change has serious implications when you are talking about treating someone with a monoclonal antibody.”
This systematic study on the huge pool of antibodies is a massive undertaking. This study provides a framework to understand, on a global scale, which antibodies are effective (or not) against which variants. This information will be key in narrowing the pool of antibodies from which to advance to further study. “Every member of our 25-person lab pitched in,” says Saphire.
With this new study, the CoVIC is closer to developing more potent antibody therapies that could pack a punch against SARS-CoV-2 variants. More powerful antibody therapies might also be effective at a lower dose, says Schendel, making them a practical option for distribution in countries where medical care is less accessible.
The CoVIC team is now working with partners on animal protection studies. Other CoVIC researchers are working to understand how neutralizing antibodies coordinate with immune system responses.
Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study. Science, 23 September 2021.