An international research team discovered the first human antibody to effectively neutralize two types of hantaviruses in animal models, according to a study published online Mar. 16 in Science Translational Medicine.
Based on their initial results, the antibody appears to be a promising candidate for developing a “pan-hantavirus” therapy to protect against outbreaks caused by multiple types of known or emerging hantaviruses.
Collectively, hantaviruses cause about 50,000 severe and often fatal infections worldwide in people each year. While humans are typically infected by contact with rodents, the viruses also can be transmitted by direct person-to-person contact. No approved therapies are currently available for treating hantavirus infection.
Rodent-borne hantaviruses are grouped into two distinct viral families, commonly called “Old World” and “New World” hantaviruses, and cause two types of disease. Hemorrhagic fever with renal syndrome (HFRS) is caused by Old World viruses found mostly in Europe and Asia, while New World viruses cause hantavirus cardiopulmonary syndrome (HCPS) and are typically found in North and South America.
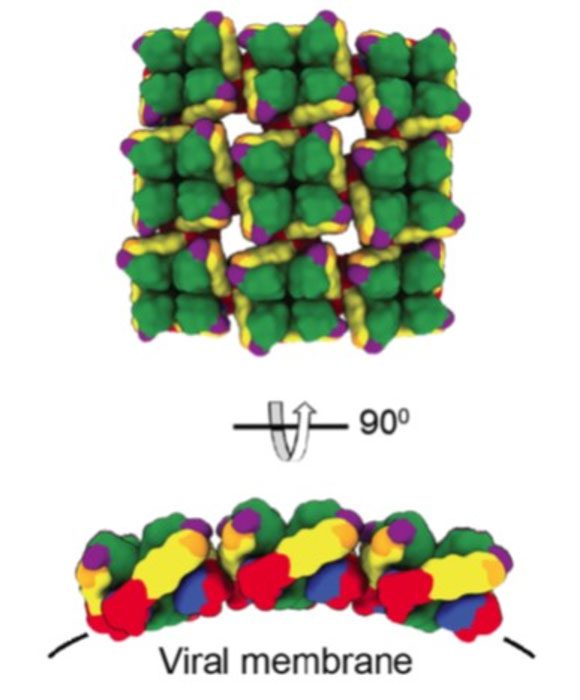
In this paper, scientists describe isolating several human monoclonal antibodies, or mAbs, from a patient in Sweden who survived infection with Puumala virus, which causes HFRS in humans. Through initial screening in cell culture, the team identified several mAbs that effectively neutralized both Old World and New World hantaviruses.
Next, the researchers tested a mAb that looked especially promising in two different animal models. They challenged hamsters and voles with Puumala (Old World) virus and Andes (New World) virus, and found that this single antibody afforded broad protection to both groups.
“This is the first monoclonal antibody to demonstrate cross-protective efficacy against divergent Old and New World hantaviruses,” said Andrew S. Herbert, Ph.D., branch chief for viral immunology at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). Herbert served as co-senior author of the paper and directed the Andes virus challenge studies at the Institute.
According to the authors, hantavirus outbreaks in Sweden, Argentina, and the United States over the past two decades highlight the public health risks posted by these viruses. The frequency of these and other emerging disease outbreaks is expected to grow in concert with animal habitat loss and climate change.
“The lack of FDA-approved or emergency use-authorized vaccines and therapeutics represents a critical gap in our preparedness for a public health emergency caused by a large hantavirus outbreak,” they conclude.
Human antibody recognizing a quaternary epitope in the Puumala virus glycoprotein provides broad protection against orthohantaviruses. Science Translational Medicine, 16 March 2022.
Summary: Rodent-borne orthohantaviruses are globally distributed zoonotic viruses causing hantavirus cardiopulmonary syndrome (HCPS) or hemorrhagic fever with renal syndrome (HFRS). Currently, no FDA-approved vaccines or antiviral therapies exist to prevent or treat severe and potentially fatal disease caused by emerging hantaviruses. Mittler et al. describe the isolation of human monoclonal antibodies (mAbs) against the spike protein complex of the hantavirus Puumala virus (PUUV) from PUUV-experienced donors recovering from HFRS. The panel of 135 mAbs displayed varying cross-reactivity and neutralizing activity against viruses from different hantavirus clades and targeted distinct parts of the viral spike. The mAb ADI-42898 blocked viral entry by seven different hantaviruses and protected Syrian hamsters challenged with the HCPS-causing Andes virus or bank voles challenged with the HFRS-causing PUUV. These findings suggest that mAbs may have utility as therapeutics for treating hantavirus disease.
Sources: USAMRIID, Science Translational Medicine