A Rare Outbreak with Historic Echoes
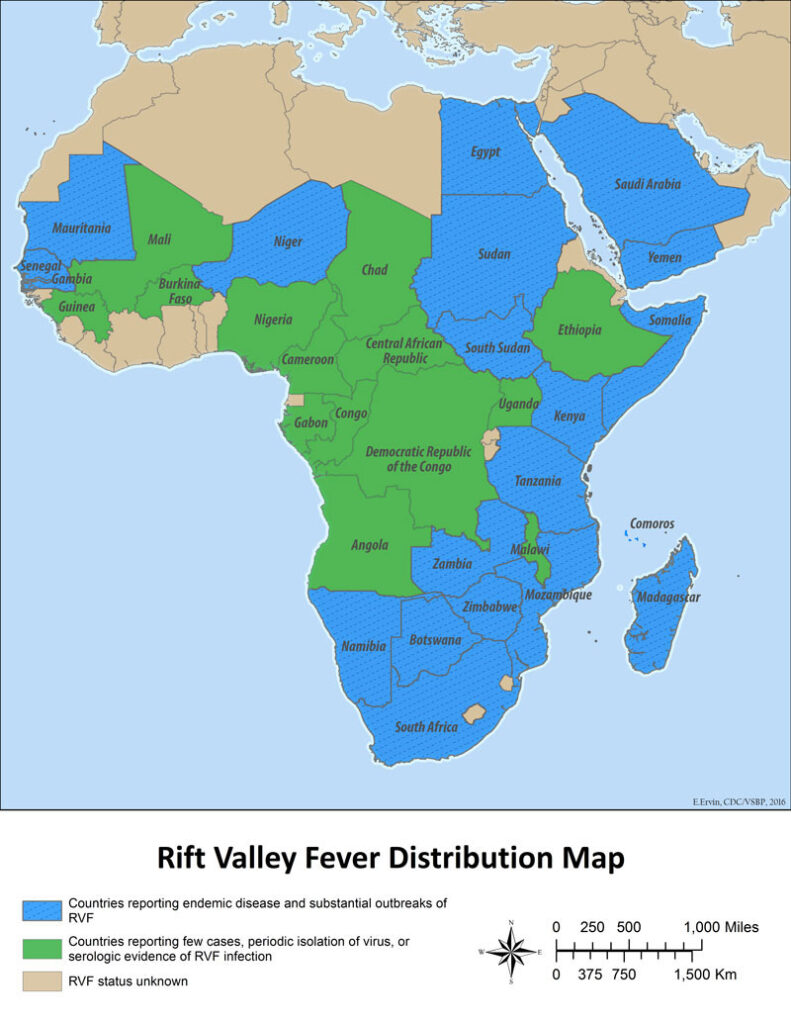
Senegal is confronting its worst outbreak of Rift Valley Fever (RVF) in decades, with at least 17 confirmed deaths and over 100 infections since late September 2025. The country’s Ministry of Health declared the outbreak on September 21, reporting that most cases occurred in northern livestock-producing regions, including Saint-Louis, Matam, and Louga, areas already struggling with heavy seasonal rains and flooding.
The Institut Pasteur de Dakar has since published a molecular analysis of the virus, confirming that this outbreak belongs to lineage H — a strain previously seen in Senegal’s 2020 and 2022 clusters and in Mauritania’s 2020 outbreak. The study, released on Virological.org, underscores that the virus has likely persisted locally rather than being newly introduced, pointing to ongoing environmental and ecological drivers.
Viral Biology and Transmission
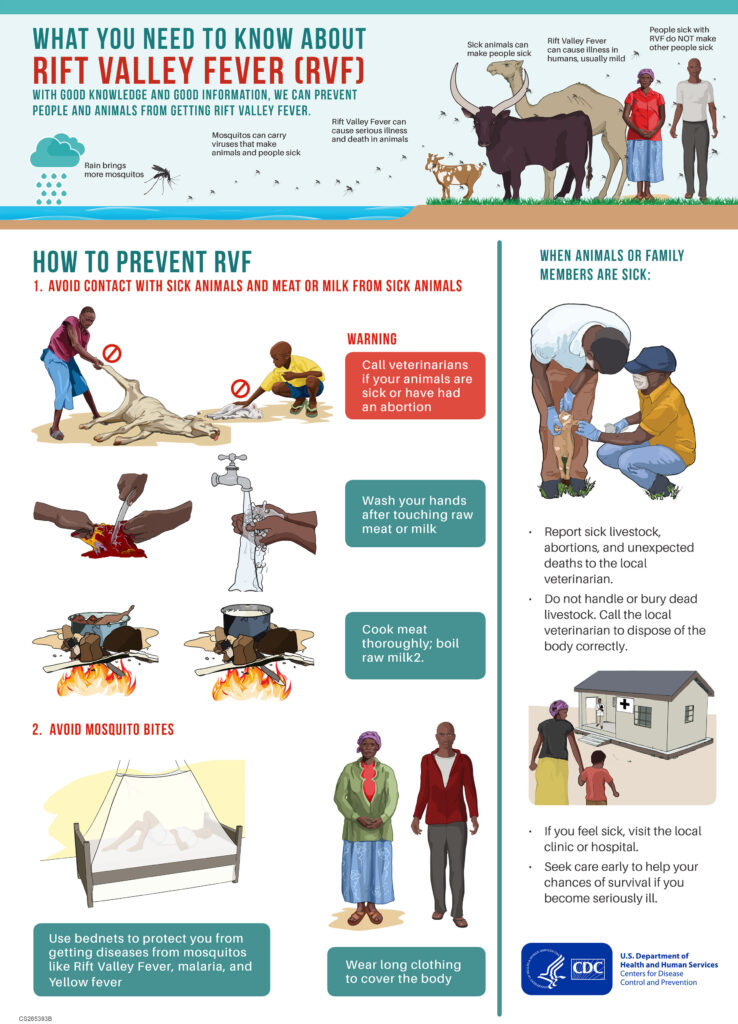
RVF is caused by a Phlebovirus in the Bunyaviridae family, a pathogen recognized in the United States as a Select Agent due to its potential for severe human disease and economic disruption in livestock. Transmission occurs mainly through mosquito bites or contact with infected animal tissues, particularly during slaughter or birthing. Although human-to-human transmission has not been confirmed, the virus can cause a wide range of symptoms — from mild fever to hemorrhagic fever, encephalitis, or vision loss.
The Centers for Disease Control and Prevention (CDC) issued a Level 1 Travel Health Notice for Senegal on October 10, advising travelers to take standard precautions such as avoiding mosquito bites and contact with livestock.
Climate and the Ecology of Outbreaks
Experts link this outbreak to unusual weather patterns — alternating heavy rains and heat — which have expanded mosquito breeding grounds and intensified the virus’s spread. Such patterns reflect broader climate-driven disease emergence, where vector populations flourish in newly suitable environments.
Senegal’s last major epidemic in the 1980s killed over 200 people, and the country has since faced sporadic reappearances. But the scale of the 2025 resurgence, along with simultaneous RVF spikes in Mauritania and other Sahelian regions, indicates that regional environmental shifts may be sustaining endemic transmission.
Genomic Clues from the Institut Pasteur
The genomic sequencing performed by the Institut Pasteur de Dakar revealed high conservation across viral genomes — over 99% nucleotide identity with earlier local isolates — confirming that this is not a new introduction but continued local persistence. Notably, the researchers identified three mutations in the L polymerase gene (D11N, M120T, Y1852H) that could subtly modify replication efficiency, though vaccine-relevant regions remain unchanged.
Such findings highlight how molecular epidemiology and One Health surveillance — integrating data from human, animal, and environmental health — can illuminate the dynamics of emerging zoonoses in Africa’s rapidly changing ecosystems.
Public Health and Biosecurity Significance
Rift Valley Fever’s dual impact on human and animal health makes it a sentinel event for zoonotic spillover preparedness. Its inclusion as a Select Agent under U.S. regulations reflects concerns over its potential misuse, agricultural consequences, and need for high-containment laboratory handling.
From a public health and national security standpoint, RVF underscores how infectious disease threats anywhere can pose global risks — disrupting food systems, trade, and stability. Strengthening veterinary surveillance, rapid diagnostics, and cross-border cooperation are critical to reducing both human morbidity and economic loss.
Containment and Next Steps
Senegal’s Ministry of Health, in collaboration with the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), is ramping up livestock vaccination campaigns and vector control operations. Field teams from Institut Pasteur de Dakar are also conducting One Health investigations to measure mosquito infection rates and livestock amplification cycles.
Although the current outbreak appears localized, experts caution that the Sahel’s ecology is increasingly conducive to recurring RVF activity. International health partners are closely monitoring for possible spread to neighboring regions and emphasizing the importance of long-term climate resilience strategies in outbreak prevention.
Sources and Further Reading:
CDC: Travel Health Notice on Rift Valley Fever in Senegal
Africa News: Senegal reports 17 deaths in rare Rift Valley Fever outbreak
AP News: Viral disease outbreak in Senegal kills 17 people
DevDiscourse: Senegal battles rare Rift Valley Fever outbreak: 17 dead
Institut Pasteur de Dakar / Virological.org: Molecular characterization of Rift Valley fever virus from the 2025 outbreak in northern Senegal
CDC Resource Graphics: