Occasionally, warfighters may have to rely on “gut” instincts to make the right decisions to defeat the enemy. Well, now an artificial piece of gut could help in the effort to develop autonomously functioning cells that track and defeat biological agents within the body.
The latest effort of DTRA CB/JSTO work managed by Dr. Ilya Elashvili has developed a first-ever, three-dimensional (3-D) synthetic intestine that works more like a real intestine. This synthetic intestine serves as a testbed and allows researchers to replace animal models in the effort to further perfect “smart” bacteria, autonomous high-order functioning cells that sense and defeat infectious diseases and other maladies that could pose a threat to the warfighter.
The work builds on previous DTRA-funded successes by this team that developed “smart” bacteria that targeted both human pathogens and cancer cells.
A research team led by Dr. William E. Bentley from the University of Maryland is working on understanding and manipulating biological signaling processes through acquiring and redesigning a trove of involved modular components. These components then can be rewired to create “smart” bacteria for controlling these processes for user-specified performance in dynamic and heterogeneous environments. Of key importance in this work are the signals that unmask infectious agents – what they are and when they occur. This is especially true in the gastrointestinal (GI) tract where commensal bacteria, food, and host factors co-exist in a dynamic and complex environment.
The GI tract is often the best point of entry for pathogens, yet few tools exist that enable its study. Animal models are both costly and inaccurate – finding a single pathogenic bacterium within a sea of commensal organisms inside the body is a daunting challenge. This team is developing a suite of technologies that serve to “open” to the GI landscape so that pathogen discovery and modes of entry to our bodies can be revealed.
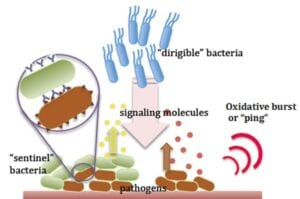
In two articles, they show how a microscale GI “laboratory-on-a-chip” can recreate the native GI tract with its complex geometry with many niches for bacterial occupation. They also show how they might register a “ping,” or a molecular beacon that the GI tract emits when a pathogen attacks.
As described in the Biotechnology and Bioengineering article, “Synthetic small intestinal scaffolds for improved studies of intestinal differentiation,” the researchers have constructed a 3-D scaffold on which intestinal cells are grown, recreating the native protrusions (villi) extending from the intestinal wall. They showed how the fabrication methodology is tailored to provide a porous structure through which nutrients and immune cells and molecules are transported. The GI tract’s native intestinal cells are present in a single layer providing a selective barrier to pathogens and nutrients. Their assembly onto the 3-D scaffold preserves their function and enables the identical geometry for nutrient transport.
Synthetic in vitro intestinal models provide a standardized testbed, much better than animal models, that could enable controlling of many different parameters simultaneously, such as host cell density, nutrient availability, pH, and fluid dynamics. Multiple in vitro models can also be run at the same time, reducing the expense and ethical issues raised by using numerous live animals. It is noteworthy in this regard that many intestinal processes are difficult to control using in vivo intestinal models, particularly the response of epithelial cells to specific environmental cues. Synthetic in vitro intestinal models can potentially enable improved studies of intestinal function in an ethical and well controlled manner, particularly for studies of cellular growth and proliferation, drug absorption, and host-microbial interactions.
In the Advanced Functional Materials article, “Enzymatic Writing to Soft Films: Potential to Filter, Store and Analyze Biologically Relevant Chemical Information,” the team focused on the natural “ping” that exists when bacteria and other pathogens invade human cells. The human response is often an oxidative burst, an emission of oxygen radicals and hydrogen peroxide meant to kill the pathogen and also signal the native immune system to respond in a more comprehensive manner. The burst serves as both a signal to recruit pathogen-attacking bacteria and as a location marker. In this article, they show that the oxidative burst can be deciphered using a biological redox capacitor sensor they constructed last year. Here they show that systems-level information can be extracted from the sensor. That is, electronic devices use dynamic inputs and real time frequency responses to extract information.
Biological assays are typically performed one test at a time and only after significant sample preparation. Because the oxidative burst involves electrochemically active molecules, this team has developed a first of its kind methodology to record in real time, the minute and diffuse oxidative responses of biological systems (reviewed in the Analyst article, “Redox-capacitor to connect electrochemistry to redox-biology”).
This effort builds on previous work by the team to manipulate E. coli to sense, fight, and defeat the common disease causing human pathogen, Pseudomonas aeruginosa, while leaving good bacteria unharmed. The team also successfully engineered E. coli bacteria that were able to sense, track, home in on cancer cells, and synthesized a preplanned compound when the cancer cell population exceeded a preset threshold (see “Eye in the Sky Launched As ‘Dirigible’ Looks to Fight Diseases” in the Feb-Mar 2013 edition of JSTO in the News).
The effort to develop “smart” bacteria will be greatly enhanced by these new advances to develop the standardized 3-D model where these bacteria can be tested ultimately to provide better protections for the warfighter.
Article courtesy of DVIDS and DTRA CB/JSTO. POC: Dr. Ilya Elashvili
Image: Schematic depicts one of the conceptual autonomously functioning antimicrobial systems. This re-programmed sentinel-dirigible system is comprised of “sentinel” cells that emit a signal upon sensing infectious agents and “dirigible” cells that are motility programmed to migrate near the signal and synthesize and deliver drugs to kill the pathogen. The human body’s “ping” or oxidative burst serves as a signal to the location of the invading pathogen. Image courtesy: Dr. William E. Bentley, University of Maryland.


