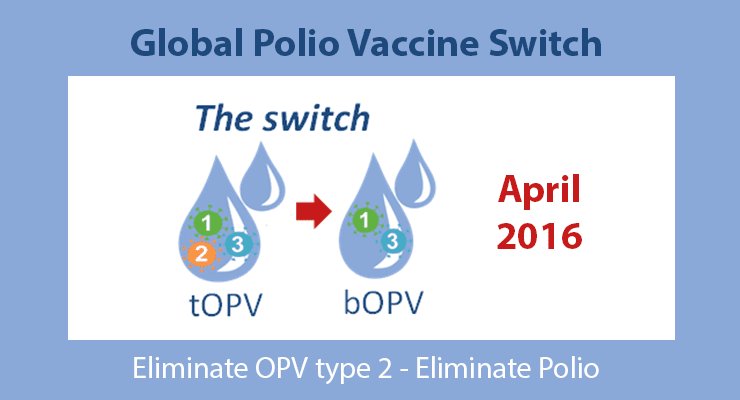
A landmark decision made in Geneva last month will pave the way for a worldwide switch from trivalent to bivalent oral polio vaccine, beginning in April 2016.
The Strategic Advisory Group of Experts on Immunization (SAGE), part of the World Health Organization, recently confirmed the globally coordinated withdrawal next spring of the type 2 component in the oral poliovirus vaccine (OPV).
SAGE’s landmark decision follows the endorsement by the World Health Assembly (WHA) in May 2015, when Ministers of Health from 194 member states adopted a resolution on the global effort to eradicate polio.
The Group encouraged all countries and the partners of the Global Polio Eradication Initiative (GPEI) to begin to intensify their preparatory efforts to switch from trivalent oral polio vaccine (tOPV) to bivalent OPV (bOPV), which is scheduled to occur from 17 April to 1 May 2016.
SAGE concluded that significant progress had been made since its last meeting in April 2015, with no cases in Africa since August and over a year having passed since the last case in the Middle East was seen, strengthened surveillance and more children being reached with vaccines in key areas of Pakistan and Afghanistan.
Withdrawing OPV type 2 is a crucial part of the polio endgame strategy, in order to eliminate the very rare cases of vaccine associated paralytic polio (VAPP) or circulating vaccine derived polioviruses (cVDPVs).
The type 2 component of OPV accounts for 40% of VAPP cases, and upwards of 90% of cVDPV cases. By contrast, wild poliovirus type 2 has not been detected anywhere since 1999 and the Global Commission for the Certification of Poliomyelitis Eradication (GCC) declared this strain globally eradicated at its meeting in September 2015.
The withdrawal of type 2 OPV will ultimately eliminate the risk of emergence of new cVDPV2s in the future, and will also prevent upwards of 200 cases of VAPP that currently occur each year as a result of the type 2 component in trivalent OPV.
Learn more about these efforts at the Global Polio Eradication Initiative.