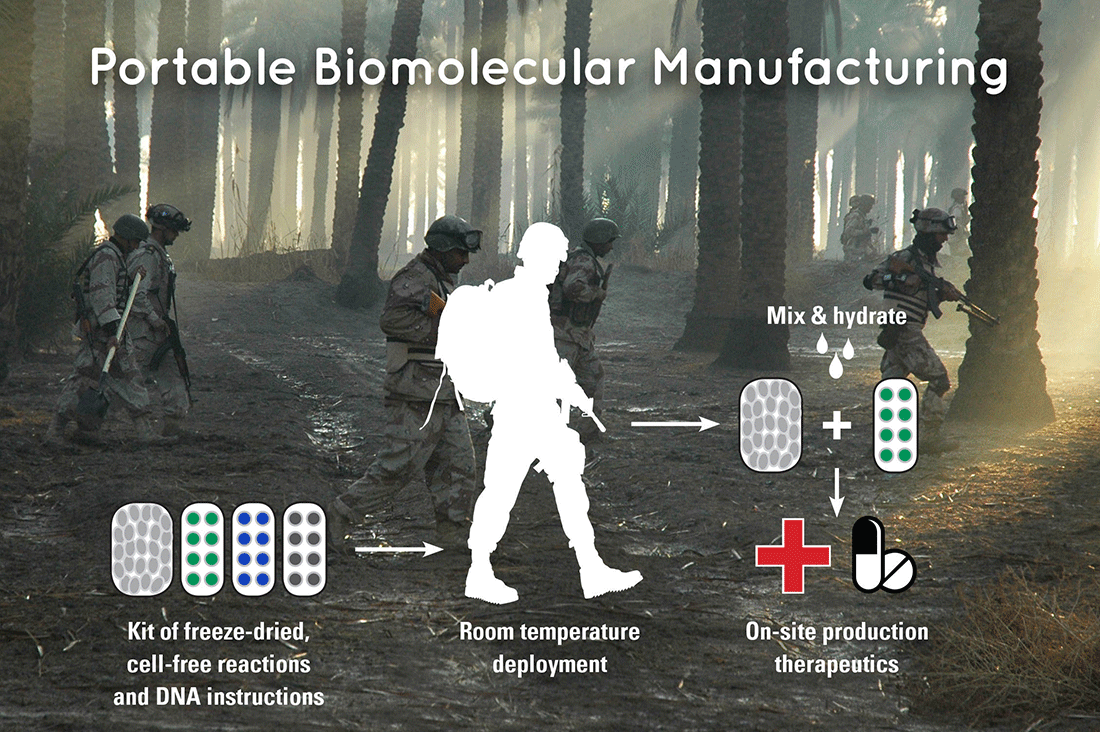
Researchers led by Dr. James Collins, from the Massachusetts Institute of Technology (MIT), have developed a novel cell-free approach for the on-demand manufacturing of therapeutics and biomolecules with the addition of water.
Funded by the Defense Threat Reduction Agency’s Joint Science and Technology Office and managed by DTRA’s Dr. Ilya Elashvili, the project builds upon earlier DTRA-funded efforts.
Previously, researchers focused on freeze-drying cell-free (FD-CF) protein expression machinery onto paper, creating a diagnostic platform that retains the protein synthesis capability of live cells, while remaining abiotic, sterile and portable. Combined with “toehold switch” RNA sensors, the researchers demonstrated proof-of-concept, low-cost diagnostics for thirteen pathogens, including the Zika virus.
The new system expands this technology beyond diagnostics to portable bio-manufacturing and is also rooted in FD-CF protein expression machinery utilizing dry reaction pellets. Combined with the DNA encoding, the instructions for biosynthesis, these pellets can be transported and stored for up to a year at ambient temperature. When water is added at the point-of-care, biosynthesis begins and yields the therapeutic within one to two hours.
The lack of accessibility to modern therapeutics has prompted the recent proposal of various systems which are often costly and require highly trained personnel and large, specialized equipment. However, the low-cost FD-CF bio-manufacturing system solves the accessibility issues while remaining easy to use. The system also offers the general benefits and flexibility inherent to in-vitro biosynthesis.
The MIT-team demonstrated this flexibility by producing a wide range of biomolecules, including antimicrobial peptides (AMPs), antibodies, enzymes for small-molecule therapeutics synthesis and vaccines. The team began with the production, purification and functional validation of AMPs, which have broad activity against bacteria, viruses, fungi and cancer cells. To produce the AMPs, the researchers utilized the standard FD-CF format by adding water to reaction pellets containing the DNA templates for ten different AMPs and then incubating at body temperature for two hours.
Antibodies against 15 specific targets were also generated, which can be used directly for protein-based diagnostics and therapeutics or conjugated to other proteins produced using the system in order to expand their functions depending on an end-user’s preference.
Using FD-CF, the reconstitution of a complex, five-enzyme pathway for the biosynthesis of the high-value, small-molecule compound violacein, was also presented. Violacein has antimicrobial, antitumor, and antiparasitic properties. This procedure demonstrated the expansion of the FD-CF platform beyond peptide and protein production to the biosynthesis of small molecules as the end product.
The team also verified the expression of vaccines for botulinum, anthrax and diphtheria. Due to its sensitivity to both heat and freezing, the researchers chose to subject the diphtheria antigen to further testing. Using FD-CF reagents, two versions of the vaccine, DT5 and DT6, were successfully produced and interacted with commercial anti-diphtheria antibodies. Next, the researchers scaled up the production of the DT5 antigen (33 human doses) and tested it in mice, which resulted in the induction of immunity within five weeks of injection.
A platform for the portable biosynthesis of biomolecules on-demand provides new capabilities for the Department of Defense and may have significant global health benefits. The production of essential therapeutics and vaccines would greatly reduce the logistical and economic burden when deploying warfighters to underdeveloped environments and improve overall troop safety and health.
For more information, read the Cell article, “Portable, On-Demand Biomolecular Manufacturing.”
Article courtesy of the Defense Threat Reduction Agency’s Chemical and Biological Technologies Department, edited for context and format by Global Biodefense.


