A current Defense Threat Reduction Agency (DTRA) research effort, conducted by the U.S. Army Research Institute for Chemical Defense (USAMRICD), is exploring the decontamination properties of Veriox®, a topical antimicrobial, anti-infective and disinfectant to counter the deadly nerve agent VX.
Veriox® is under development for use in hospitals for medical device sterilization, surface disinfection and advanced wound care. The same properties that make it useful in these situations may also mean that it could be used to treat warfighters after exposure to a chemical weapon.
Dermal or inhalation exposure to VX, like most chemical weapon nerve agents, may result in muscle paralysis, shortness of breath, seizures and death. To counter this threat, our warfighters need a reliable treatment option for both broken and unbroken skin.
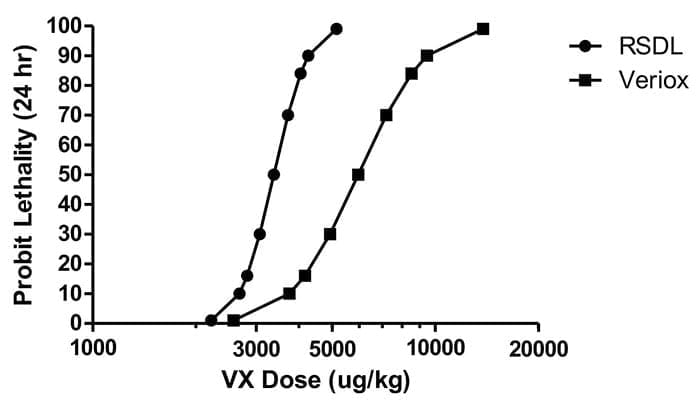
While the Department of Defense currently uses the Reactive Skin Decontamination Lotion (RSDL) for broad-spectrum agent elimination on unbroken skin, a capability gap exists for treating chemical agent exposure to large affected areas or open wounds. This need has led researchers from DTRA to pursue new personnel decontamination therapeutics.
Recent USAMRICD studies have shown that the median lethal dose of VX in Veriox®-treated animals is 1.8-fold higher than in RSDL-treated animals. While preliminary studies demonstrate Veriox® provides a significant reduction in lethality from nerve agents, in-depth efficacy studies are needed to fully assess the compound. If successful, Veriox® could provide an alternative dermal capability for military personnel, particularly for open wound and whole-body decontamination.
The USAMRICD team recently published their findings in the report, “USAMRICD-TR-16-06, Evaluation of Veriox® as a Skin Decontamination Product after Dermal Exposure to the Nerve Agent VX.”
DTRA is also working with the Edgewood Chemical and Biological Center on a parallel decontamination effort utilizing zirconium hydroxide (Zr(OH)4). This effort has demonstrated dermal efficacy equal to or greater than RSDL against several chemical weapon agents, including VX, sulfur mustard and soman, when tested on pig and artificial skin. In addition, multiple endeavors to explore novel formulations of medical decontamination capabilities for open-wound treatment after exposure are underway.
Article adapted from original by the Defense Threat Reduction Agency. Edited for context and format by Global Biodefense.