Many health security threats involve bacteria or chemicals for which clinical trials cannot be performed in humans. To overcome this hurdle, U.S. Biomedical Advanced Research Projects Agency (BARDA) works closely with the Food and Drug Administration on a path for approval using the Animal Rule. BARDA also invests in reduced business risk and costs not just by providing non-dilutive funding and deep technical assistance but also by focusing on products that could have multiple uses and commercial uses.
In some cases, BARDA has sponsored development for new indications of approved products like Seizalam and Silverlon. Midazolam was an approved sedative and became the first approved as an anti-seizure drug to treat prolonged seizures caused by chemical exposure. Silverlon was cleared initially as a silver-impregnated wound dressing and became the first product cleared in the U.S. for use in treating chemical burns from mustard gas.
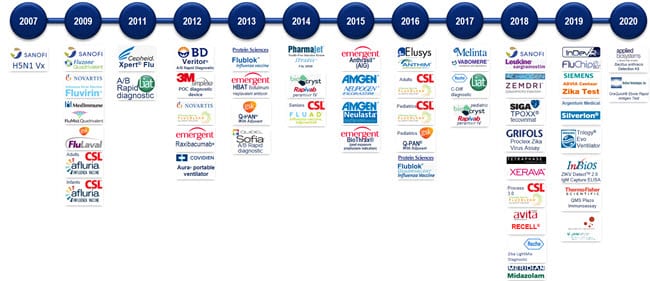
Description
| # | Medical Countermeasure | Threat Area | Company Sponsor | Year |
|---|---|---|---|---|
| 1 | H5N1 vaccine Antigen-alone formulation | Influenza | sanofi pasteur | 2007 |
| 2 | Fluzone® H1N1 influenza vaccine adult | Influenza | sanofi pasteur | 2009 |
| 3 | Fluvirin® H1N1 influenza vaccine | Influenza | Novartis | 2009 |
| 4 | FluMist® H1N1 influenza vaccine | Influenza | MedImmune | 2009 |
| 5 | FluLaval® H1N1 influenza vaccine | Influenza | GlaxoSmithKline | 2009 |
| 6 | Afluria® H1N1 influenza vaccine infants | Influenza | Commonwealth Serum Laboratories | 2009 |
| 7 | Afluria® H1N1 influenza vaccine adult | Influenza | Commonwealth Serum Laboratories | 2009 |
| 8 | XPERT FLU® H1N1 Influenza POC diagnostic | Influenza | Cepheid | 2011 |
| 9 | LIAT®Influenza A/B Rapid diagnostic Influenza A/B Rapid diagnostic | Influenza | Iquum / (Roche) | 2011 |
| 10 | Veritor® Influenza A/B Rapid diagnostic | Influenza | Becton Dickinson | 2012 |
| 11 | Simplexa® Point-of-care diagnostic device | Influenza | Focus/3M | 2012 |
| 12 | Flucelvax® Seasonal cell-based influenza vaccine | Influenza | Novartis | 2012 |
| 13 | Aura® Next generation portable ventilator | Influenza | Covidien | 2012 |
| 14 | Raxibacumab® Anthrax antitoxin | Anthrax | GlaxoSmithKline (formerly HGS) | 2012 |
| 15 | FluBlØk® Seasonal recombinant-based influenza vaccine | Influenza | Protein Sciences | 2013 |
| 16 | HBAT Botulinum heptavalent antitoxin | Botulism | Emergent (formerly Cangene) | 2013 |
| 17 | Q-PAN® H5N1 Pandemic influenza vaccine with adjuvant | Influenza | GlaxoSmithKline | 2013 |
| 18 | Sophia® Influenza A/B Rapid diagnostic | Influenza | Quidel (Nanogen) | 2013 |
| 19 | PharmaJet Needleless Flu Shot Stratis | Influenza | PharmaJet | 2014 |
| 20 | Rapivab® (peramivir) Influenza antiviral drug IV | Influenza | BioCryst | 2014 |
| 21 | Anthrasil™ (AIG) Anthrax antitoxin | Anthrax | Cangene | 2015 |
| 22 | Neupogen® (filgrastrim) ARS anti-neutropenia cytokine | RAD/NUC | Amgen | 2015 |
| 23 | Neulasta (GM-CSFpeg) ARS anti-neutropenia cytokine | Rad/Nuc | Amgen | 2015 |
| 24 | BioThrax® (post-exposure prophylaxis indication) | Anthrax | Emergent | 2015 |
| 25 | Fluad (with adjuvant) Influenza vaccine for seniors | Influenza | Sequris (CSL – Novartis) | 2015 |
| 26 | Anthim Anthrax Monoclonal | Anthrax | Elusys | 2016 |
| 27 | Flucelvax Quadrivalent Influenza Virus Vaccine | Influenza | Seqrius | 2016 |
| 28 | Flucelvax Quadrivalent Influenza Virus Vaccine (Pediatric Indication) | Influenza | Seqirus | 2016 |
| 29 | Q-Pan H5N1 AS03-Adjuvanted Pandemic Influenza Virus Vaccine for Pediatrics | Influenza | GSK | 2016 |
| 30 | Flublok Quadrivalent Influenza Virus Vaccine | Influenza | Protein Sciences Corporation | 2016 |
| 31 | Vabomere (Carbavance) | BSA | MEDCO | 2017 |
| 32 | Rapivab® (peramivir) Influenza antiviral drug IV | Influenza | BioCryst | 2017 |
| 33 | Cobas LIAT C. Diff POC diagnostic | CBRN | Roche | 2017 |
| 34 | Roche LightMix Zika Molecular Diagnostic | Zika | Roche | 2017 |
| 35 | Leukine® (sargramostim) ARS anti-neutropenia cytokine | RAD/NUC | sanofi aventis | 2018 |
| 36 | ZEMDRI Plazomicin | BSA | Achaogen | 2018 |
| 37 | Procleix Zika Virus Assay | Zika | Hologic/Grifols | 2018 |
| 38 | Arestvyr®ST-246 (Tecovirimat) Smallpox antiviral drug | Smallpox | SIGA | 2018 |
| 39 | Xerava | BSA | Tetraphase | 2018 |
| 40 | Flucelvax Process 3.0 | Influenza | Seqirus | 2018 |
| 41 | Seizalam | Chemical | Meridian | 2018 |
| 42 | RECELL | Burn | Avita | 2018 |
| 43 | QMS Plazomicin Immunoassay | BSA | Achaogen (Thermo Fisher) | 2018 |
| 44 | FluChip-8G Influenza A+B Assay | Influenza | InDevR | 2019 |
| 45 | InBios International Manual Zika IgM assay | Zika | InBios | 2019 |
| 46 | ADVIA Centaur® Zika test | Zika | Siemens | 2019 |
| 47 | Trilogy Evo Universal (K181170) ventilator | Influenza | Phillips | 2019 |
| 48 | Silverlon | Chemical | Argentum Medical | 2019 |
| 49 | IMVAMUNE® Smallpox MVA Vaccine | Smallpox | Bavarian Nordic | 2019 |
| 50 | Applied Biosystems™ Bacillus anthracis Detection Kit | Anthrax | Applied Biosystems | 2019 |
| 51 | OraQuick Ebola Rapid Antigen | Ebola | OraSure | 2019 |
Over the past 12 years, BARDA has partnered with more than 300 companies, from micro and small businesses to global pharmaceutical corporations. Learn more about partnering with BARDA and start a conversation with them by requesting a meeting.