Scientists at Scripps Research analyzing coronavirus antibodies found more antibodies that recognize other, related viruses in people who had been infected with COVID-19.
Getting sick with a common cold doesn’t make you immune to COVID-19, but a COVID-19 infection might, at least temporarily, boost the number of antibodies you have against common cold-causing coronaviruses and the SARS-CoV-1 and MERS-CoV viruses, all of which are closely related. Scientists at Scripps Research have now characterized coronavirus antibodies isolated from 11 people to reveal how COVID-19 impacts the immune system’s ability to recognize other coronaviruses.
“Getting a better understanding of how immunity against this broad family of coronaviruses changes with COVID-19 infection is an important step toward developing better coronavirus vaccines, both for COVID-19 and for future, related pathogens,” says Andrew Ward, PhD, professor of Integrative Structural and Computational Biology at Scripps Research and senior author of the new paper, published online this week in Science Advances.
The SARS-CoV-2 virus that causes COVID-19 is just one in a large and diverse family of coronaviruses. A few of its relatives are equally contagious and virulent — causing Middle East respiratory syndrome (MERS) and the 2002-2004 SARS outbreak — while others, considered common cold viruses, cause much milder symptoms. Overall, many of these coronaviruses have only one quarter to one half of their genetic material in common with SARS-CoV-2, but individual sections of the viruses’ structures—most notably the spike protein that juts out of each coronavirus—are considered relatively similar between family members.
Since the outset of the COVID-19 pandemic, scientists have wondered whether people’s previous exposure to those common cold viruses impacts their immunity to SARS-CoV-2 and, likewise, whether infection with COVID-19 might change how the immune system recognizes the more common coronaviruses. The immune system’s antibodies against one coronavirus spike protein could, potentially, also recognize other similar spike proteins as disease-causing.
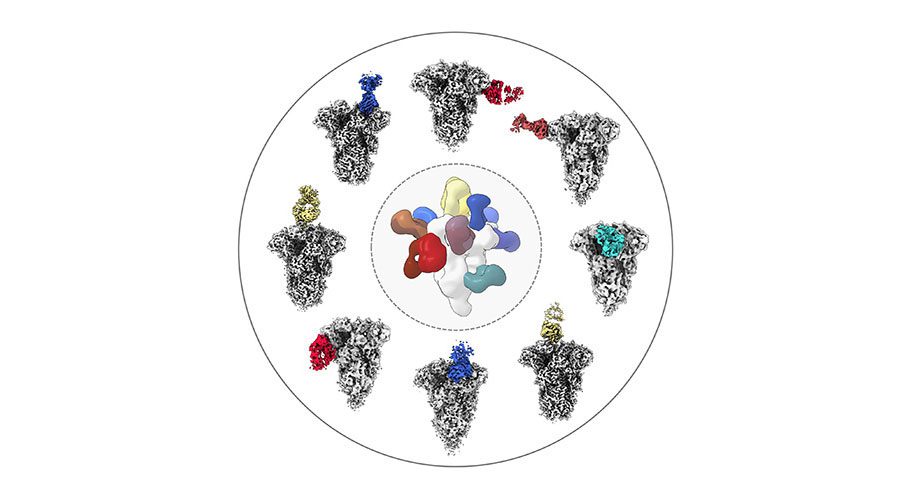
In the new study, Ward’s group studied serum samples from eleven people. Eight of the samples dated to before the COVID-19 pandemic to ensure the donors had never been exposed to the SARS-CoV-2 virus, while three samples were from donors who recently had COVID-19. In each case, the researchers measured how strongly the samples reacted to isolated spike proteins from different coronaviruses— OC43 and HKU1, both associated with common colds, along with SARS-CoV-1, MERS-CoV and SARS-CoV-2.
Not surprisingly, only the serum from recovered COVID-19 patients reacted to the SARS-CoV-2 spike proteins. However, these COVID-19 patient samples also reacted more strongly than the pre-pandemic samples to the other spike proteins as well.
“Most people have this baseline immunity to common coronaviruses and exposure to SARS-CoV-2 increases the levels of these antibodies,” says Scripps Research postdoctoral research associate Sandhya Bangaru, the first author of the new paper.
Ward, Bangaru and their colleagues performed high-resolution structural studies on serum antibodies from three of the healthy donors and the two COVID-19 patients to determine where on the spike proteins each antibody attached. They found that most coronavirus antibodies from before the pandemic recognized a section of the OC43 and HKU1 spike proteins known as the S1 subunit, which tends to vary greatly between coronaviruses. In COVID-19 patient samples, however, the researchers identified a broader swatch of antibodies, including ones that recognized the S2 subunit—which varies less between different coronaviruses. Indeed, some antibodies from the COVID-19 patients not only bound to the common cold coronaviruses, but to SARS-CoV- and MERS-CoV spike proteins as well.
“The end goal of this would be to rationally design vaccines that can recognize many different coronaviruses,” says Bangaru. “Our results reveal certain conserved patches on the S2 subunit targeted by antibodies naturally induced during infection, which we want to focus on.”
Since these studies were done directly on serum antibodies, the researchers don’t know whether the presence of these antibodies, in any of the cases, is enough to provide full immunity to coronaviruses in the more complex setting of the human immune system. The increased ability of convalescent sera to react to common coronaviruses appears to be the result of both increased production of new antibodies that can recognize several coronaviruses and also an increase in levels of pre-existing antibodies that are specific to each virus. However, it is not clear how much each of these phenomena contribute to the overall increase and how they would influence the natural course of COVID. In the future, they’d like to compare antibodies from the same individuals pre- and post-infection with COVID-19.
“Our work provides a baseline characterization of people’s antibody responses to endemic coronavirus prior to SARS-CoV-2 exposure but there are a lot of open questions,” says Bangaru. “We hope this leads to a lot more research.”
This work was supported by funding from the National Institute of Allergy and Infectious Diseases Center for HIV/AIDS Vaccine Development (UM1 AI144462, R01 AI127521, R01 AI157155), the Bill and Melinda Gates Foundation (OPP1170236 and INV-004923), an amfAR Mathilde Krim Fellowship in Biomedical Research (no. 110182-69-RKVA), and a Rubicon postdoctoral grant (no. 45219118) from the Netherlands Organization for Scientific Research (NWO).
Structural mapping of antibody landscapes to human betacoronavirus spike protein, Science Advances, 4 May 2022.
Summary: Preexisting immunity against seasonal coronaviruses (CoVs) represents an important variable in predicting antibody responses and disease severity to severe acute respiratory syndrome CoV-2 (SARS-CoV-2) infections. We used electron microscopy–based polyclonal epitope mapping (EMPEM) to characterize the antibody specificities against β-CoV spike proteins in prepandemic (PP) sera or SARS-CoV-2 convalescent (SC) sera. We observed that most PP sera had antibodies specific to seasonal human CoVs (HCoVs) OC43 and HKU1 spike proteins while the SC sera showed reactivity across all human β-CoVs. Detailed molecular mapping of spike-antibody complexes revealed epitopes that were differentially targeted by preexisting antibodies and SC serum antibodies. Our studies provide an antigenic landscape to β-HCoV spikes in the general population serving as a basis for cross-reactive epitope analyses in SARS-CoV-2–infected individuals.