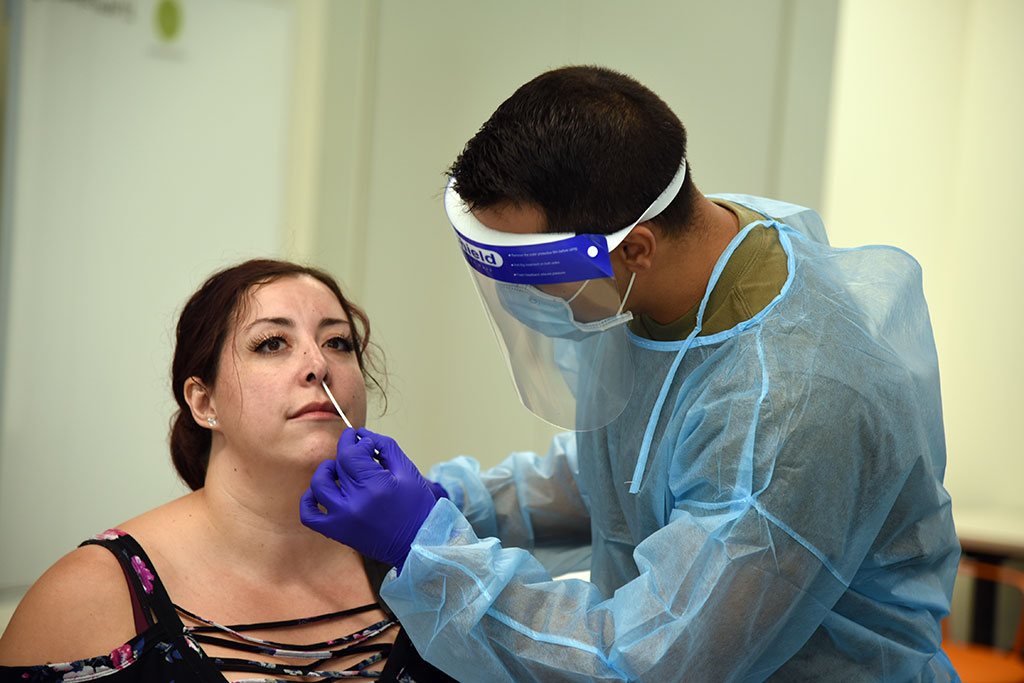
Expanded funding will support activieitechnology development and clinical testing of prototype point-of-care systems.
The National Institute for Biomedical Imaging and Bioengineering, part of the National Institutes of Health (NIH), has announced intentions to reissue PAR-17-453, Point-of-Care Technologies Research Network (POCTRN), with additional partnering NIH Institutes and Centers.
The purpose of this funding opportunity announcement is to solicit Technology Research and Development Center (TRDC) applications for the POCTRN. POCTRN’s purpose is to drive the development and application of innovative point of care, home-based, improved clinical laboratory tests and testing technologies.
The POCTRN TRDCs will merge scientific and technological capabilities with clinical need to address unmet testing, monitoring, and treatment areas.
The network of POCTRN TRDCs will have broad expertise across many research areas and will cover multiple levels of technology readiness from proof of feasibility through products and procedures used in clinical practice.
The scope of work covered within each Center will include, but are not limited to:
- Sub-project awards to support point-of-care technology development activities led by investigators
- Assessment and communication of unmet clinical needs in point-of-care testing
- Collaborations with physical scientists, computational scientists, and engineers (as well as researchers from other relevant disciplines, as appropriate) on technology development projects
- Development of external partnerships (e.g., technology, clinical, industry, and regulatory) necessary to move enabling technologies toward clinical applications
- Validation and clinical testing of prototype point-of-care devices
- Creation of training opportunities for technology developers and other stakeholders on clinical issues related to the development of point-of-care devices.
The Funding Opportunity Announcement is expected to be published in June 2022 with an application due date in Fall 2022. This opportunity is open to all applicants, including the existing POCTRN grantees as well as new applicants.
Notice of Intent to Publish a Funding Opportunity Announcement for Point-of-Care Technologies Research Network Centers (U54 Clinical Trial Optional). National Institute of Biomedical Imaging and Bioengineering (NIBIB), Notice Number: NOT-EB-22-001. First Estimated Application Due Date: September 26, 2022.