Bavarian Nordic’s smallpox vaccine is approved for use against monkeypox by the U.S. Food and Drug Administration and Health Canada.
Bavarian Nordic A/S announced today the signing of a number of supply contracts with undisclosed countries for the company’s smallpox vaccine with the aim to ensure sufficient supply to meet the requirements for vaccinating individuals at risk for monkeypox in the short to medium term.
While the terms of the agreements remain undisclosed. Bavarian Nordic is currently in dialogue with additional countries concerning supply of the vaccine to mitigate the current monkeypox outbreak and to explore opportunities for longer term collaboration to build stockpiles for future preparedness.
The company said it was making every possible effort to ensure sufficient availability of vaccines to meet the current demand in this unprecedented situation.
“The current monkeypox outbreak continues to call for a swift and coordinated response from health authorities, and we are pleased to assist more countries with supply of vaccines while we continue our dialogues with other governments to make vaccines available as fast as possible to mitigate the situation,” said Paul Chaplin, President and CEO of Bavarian Nordic.
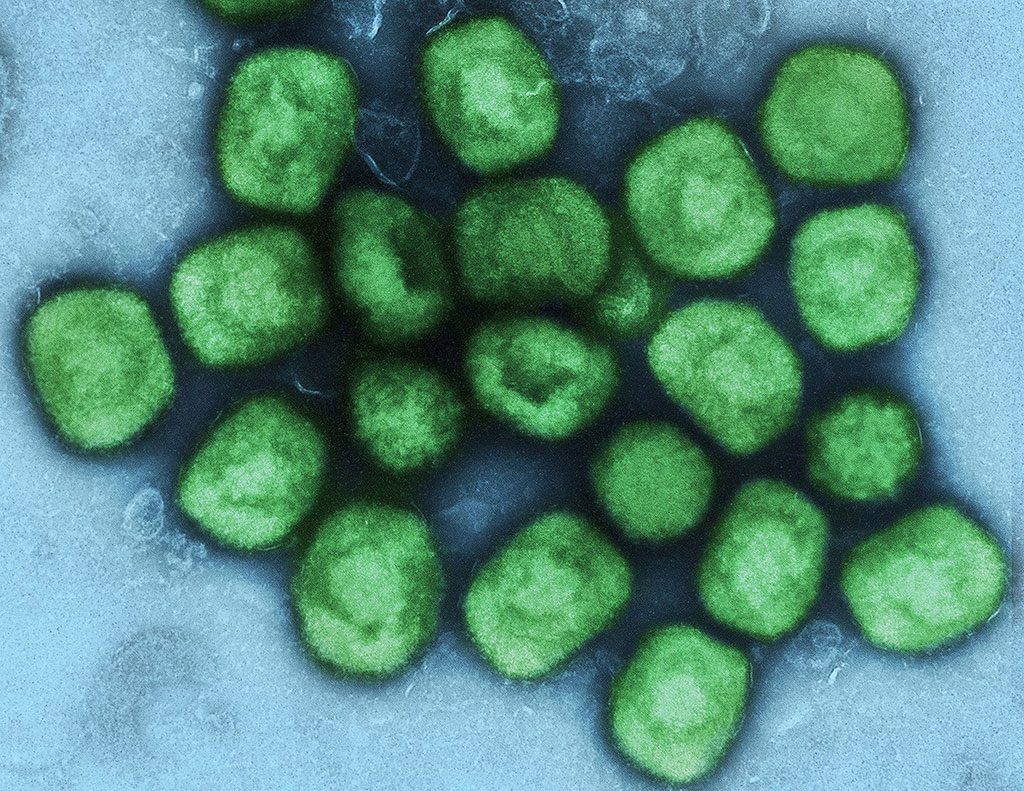
About the Smallpox/Monkeypox Vaccine
MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic (marketed as IMVANEX® in Europe, JYNNEOS® in the U.S. and IMVAMUNE® in Canada) is a non-replicating smallpox vaccine developed in collaboration with the U.S. government to ensure supply of a smallpox vaccine for the entire population, including immunocompromised individuals who are not recommended vaccination with traditional replicating smallpox vaccines. The vaccine was approved by the European Commission in 2013 for immunization against smallpox in adults aged 18 years and older and has subsequently gained regulatory approvals in Canada and the U.S. where the approval has been extended to include the monkeypox indication as the only vaccine having obtained this to-date.
Bavarian Nordic has ongoing supply contracts with USA and Canada and has delivered the vaccine to a number of undisclosed countries globally as part of their national biological preparedness. In recent years, the vaccine has been supplied in response to sporadic cases of monkeypox.
Source: Bavarian Nordic