As the official count of monkeypox cases in the U.S. hit 156 this week, the Centers for Disease Control and Prevention (CDC) moved to improve its cumbersome diagnostic testing pathway by shipping orthopoxvirus tests to five commercial laboratory companies, including the nation’s largest reference laboratories.
Clinicians previously were forced to report suspected monkeypox infections to health department officials who decide whether the cases meet criteria to undergo testing at public labs. Samples then were sent on to the CDC for confirmation. Extensive paperwork and multiple phone calls to coordinate with health officials created bottlenecks.
Adding Commercial Laboratory Testing Capability
The commercial laboratories will dramatically expand testing capacity nationwide and make testing more convenient and accessible for patients and health care providers. Health care providers will be able to use these laboratories (which include Aegis Science, Labcorp, Mayo Clinic Laboratories, Quest Diagnostics and Sonic Healthcare) by early July and testing capacity through these companies will be ramped up throughout the month. This development will facilitate increased testing, leverage established relationships between clinics, hospitals and commercial laboratories, and support our ability to better understand the scope of the current monkeypox outbreak.
“All Americans should be concerned about monkeypox cases. Thankfully we have right now the tools to fight and treat cases in America,” said Health and Human Services Secretary Xavier Becerra. “By dramatically expanding the number of testing locations throughout the country, we are making it possible for anyone who needs to be tested to do so.”
Improving Public Health Lab Monkeypox Testing Capacity
CDC, in collaboration with the Food and Drug Administration (FDA), the Centers for Medicare & Medicaid Services, and the HHS Office of the Assistant Secretary for Preparedness and Response has also been expanding the capacity in the Laboratory Response Network of public health labs. CDC has worked with the LRN to augment the number of public health laboratories that can perform the test to over 67 laboratories across 48 states and the number of weekly tests available within the LRN to over 8,000 tests per week. In addition to making testing more convenient and accessible through commercial laboratories, CDC will continue to work with state and local health departments to make the LRN process more efficient and reach out to health care providers and their state and local partners to learn more about monkeypox.
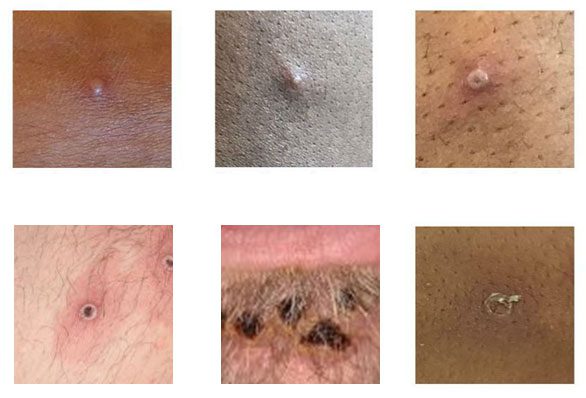
In June, CDC updated and expanded the monkeypox case definition and continues to encourage health care providers to consider testing for all rashes with clinical suspicion for monkeypox. Health care providers who see a patient with a rash that resembles monkeypox or might be more characteristic of more common infections (e.g., varicella zoster, herpes zoster, or syphilis) should carefully evaluate the patient for monkeypox (see images and links) and testing should be considered. Anyone who has risk factors for monkeypox and a new rash should seek care and testing.
Since the United States detected its first case, FDA has worked with commercial laboratories and manufacturers to make monkeypox tests more readily available to patients and providers who need them. FDA is working closely with CDC to increase production of its FDA-cleared test. FDA is concurrently exercising enforcement discretion regarding CDC’s tests, which permits hospitals to develop their own high-quality laboratory developed tests beyond the current LRN. CDC has published its FDA-approved testing protocol for any interested laboratory to use to begin testing for monkeypox. FDA has also authorized the use of additional reagents and automation to increase the testing capacity of laboratories using the CDC test.
Sources: HHS