A new diagnostic chip reduces testing time from days to one hour, allowing doctors to pick the right antibiotic the first time.
The device, developed by researchers at the University of Toronto, could transform a doctor’s ability to treat infections: a test for antibiotic resistance that works in just one hour – instead of several days.
 Each year two million people in the U.S. contract antibiotic-resistant infections, and at least 23,000 people die as a direct result.
Each year two million people in the U.S. contract antibiotic-resistant infections, and at least 23,000 people die as a direct result.
Resistant bacteria arise in part because of imprecise use of antibiotics – when a patient comes down with an infection, the doctor wants to treat it as quickly as possible. Samples of the infectious bacteria are sent to the lab for testing, but results can take two to three days. In the meantime, the doctor prescribes her patient a broad-spectrum antibiotic. Sometimes the one-size-fits-all antibiotic works and sometimes it doesn’t, and when the tests come back days later, the doctor can prescribe a specific antibiotic more likely to kill the bacteria.
PhD researcher Justin Besant and his team have designed a small and simple chip to test for antibiotic resistance in just one hour, giving doctors a shot at picking the most effective antibiotic to treat potentially deadly infections. Their work was published this week in the international journal Lab on a Chip.
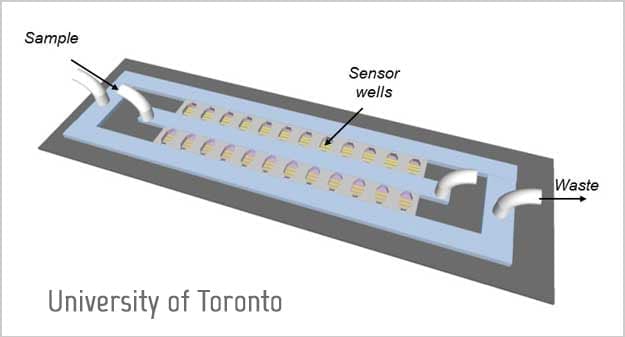
The team drew on their collective expertise in electrical and biomedical engineering to design a chip that concentrates bacteria in a miniscule space – just two nanolitres in volume – in order to increase the effective concentration of the starting sample.
They achieve this high concentration by ‘flowing’ the sample, containing the bacteria to be tested, through microfluidic wells patterned onto a glass chip. At the bottom of each well a filter, composed of a lattice of tiny microbeads, catches bacteria as the sample flows through. The bacteria accumulate in the nano-sized well, where they’re trapped with the antibiotic and a signal molecule called resazurin.
Living bacteria metabolize resazurin into a form called resorufin, changing its electrochemical signature. If the bacteria are effectively killed by the antibiotic, they stop metabolizing resazurin and the electrochemical signature in the sample stays the same. If they are antibiotic-resistant, they continue to metabolize resazurin into resorufin, altering its electrochemical signature. Electrodes built directly into the chip detect the change in current as resazurin changes to resorufin.
“This gives us two advantages,” says Besant. “One, we have a lot of bacteria in a very small space, so our effective starting concentration is much higher. And two, as the bacteria multiply and convert the resazurin molecule, it’s effectively stuck in this nanolitre droplet – it can’t diffuse away into the solution, so it can accumulate more rapidly to detectable levels.”
“Our approach is the first to combine this method of increasing sample concentration with a straightforward electrochemical readout,” says Sargent. “We see this as an effective tool for faster diagnosis and treatment of commonplace bacterial infections.”
Rapid alternatives to existing antibiotic resistance tests rely on fluorescence detection, requiring expensive and bulky fluorescence microscopes to see the result.
“The electronics for our electrochemical readout can easily fit in a very small benchtop instrument, and this is something you could see in a doctor’s office, for example,” says Besant. “The next step would be to create a device that would allow you to test many different antibiotics at many different concentrations, but we’re not there yet.”
Read the study: Rapid electrochemical phenotypic profiling of antibiotic-resistant bacteria.
Article by Marit Mitchell, Faculty of Applied Science & Engineering at the University of Toronto, edited for context and format.