The Big Idea
A new technique my colleagues and I developed that can kill deadly, multidrug-resistant bacteria in real time could be used to generate targeted therapies that replace traditional, increasingly ineffective antibiotics.
Bacteria follow the same basic genetic process that all organisms do: DNA, which contains instructions on how an organism will look and function, is copied into an intermediate form called RNA that can be translated into proteins and other molecules the organism can use.
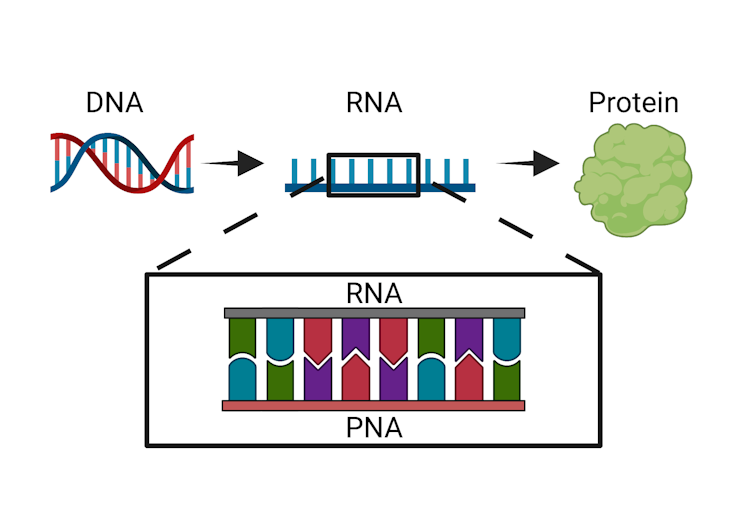
The technique we developed at the Chatterjee Lab at the University of Colorado Boulder uses a synthetic version of RNA called PNA, or peptide nucleic acid, to disrupt this basic process in bacteria. Our PNA molecule clings to the bacterial RNA, blocking it from carrying out its job. Because this molecule is a perfect match to bacterial RNA, it binds very tightly to the RNA and resists degradation. This means that it can not only escape the bacteria’s error detection processes but also prevent that RNA from being translated into proteins and other useful biological molecules. This impediment can be lethal to the bacteria.
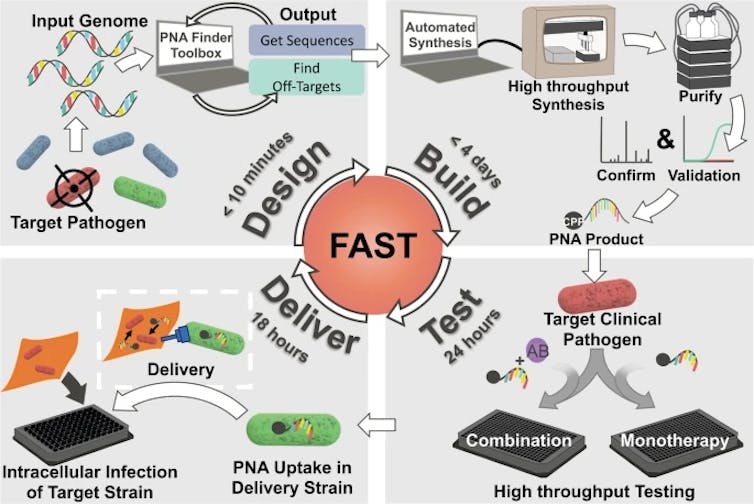
Our study, which we recently published in Communications Biology, demonstrates the therapeutic potential of a technique that can design, synthesize and test PNA treatments in under a week.
Most antibiotics aren’t specific enough to target only infectious bacteria without also destroying the body’s good bacteria. Our technology, however, uses noninfectious versions of multidrug-resistant bacteria to create highly specific molecules. By targeting just the pathogen of interest, these PNA therapeutics may avoid the harm that current antibiotics pose to the body’s good bacteria.
Why It Matters
Bacteria’s adaptation to survive current antibiotics, or antibiotic resistance, is on the rise.
Medicine’s current arsenal of treatments mostly consist of naturally occurring antibiotics that were isolated more than 30 years ago. Discovery of new antibiotics in nature has stagnated while bacteria continue to evolve and evade current treatments. And even if scientists were to find a new natural antibiotic, research shows that bacteria will begin to develop resistance within as little as 10 years, leaving us in the same predicament as before.
New types of therapies need to be considered for a post-antibiotic era, a time when our arsenal of antibiotics is no longer effective. By using a system that can target specific bacteria and be continuously modified based on emerging resistance patterns, doctors would no longer have to rely on chance discoveries. Treatments can adapt with bacteria.
What Still Isn’t Known
Although we explore multiple characteristics that determine which RNA sequences are the best targets, more research is necessary to identify the most effective PNA therapeutics against multidrug-resistant bacteria. As our study only tested our new strategy on cell cultures in the lab, we’ll also need to see how it works in living animals to maximize the effectiveness of this kind of treatment.
What’s Next
Our team is currently testing the technology in different animal models against different types of infections. We are also exploring other PNA delivery options, including adapting our bacterial delivery system to probiotic strains so it can integrate with the existing healthy bacteria population in the body.
With further development, our goal is to adapt the platform to target diseases that also use the same basic genetic processes as bacteria, such as viral infections or cancer.
Facile accelerated specific therapeutic (FAST) platform develops antisense therapies to counter multidrug-resistant bacteria. Communications Biology, 12 March 2021.
ABOUT THE AUTHOR
Kristen Eller, PhD Candidate in Chemical Engineering, University of Colorado Boulder. I am currently a PhD candidate in the Chemical Engineering program at the University of Colorado Boulder. I obtained my Master of Science degree in Chemical Engineering from the University of Colorado Boulder in 2018 and obtained my Bachelor of Science in Chemical-Biological Engineering from the Massachusetts Institute of Technology in 2016. I have been conducting multi-disciplinary research in developing novel antimicrobial therapies throughout the past 5 years, producing numerous publications including three coauthor publications on antimicrobial nanoparticles, a first author publication on anti-sense antimicrobials, and a co-first author publication on gene editing strategies to multi-drug resistant bacteria.
This article is courtesy of The Conversation.