According to the National Institute of Allergy and Infectious Diseases’ new Pandemic Preparedness Plan, the institute will direct its preparedness efforts on two fronts. First, researchers will identify “prototype pathogens”—viruses within viral families with the potential to cause significant human disease. Knowledge gained from studying prototype pathogens will also build a framework for a rapid research and product development response for other viruses within that virus family should an outbreak occur. For example, NIAID’s earlier research on SARS-CoV-1 and MERS-CoV informed rapid vaccine development for SARS-CoV-2 in 2020. The plan’s second key research focus is on priority pathogens—viruses already known to be capable of causing significant human illness or death, such as Zika virus.
NIAID aims to support critical basic and preclinical studies to characterize these prototype and priority pathogens, including understanding viral biology and structure, host-immune responses, mechanisms of immune evasion, disease pathogenesis and animal models of disease.
There are multiple virus families for which there are no available licensed vaccines, and many member viruses within families that have potential to cause significant human disease. Since it is not feasible to fully characterize the ~120 viruses known to cause human disease and develop MCMs (Medical Countermeasures) for each, selection of representative viruses from each family offers a viable pathway to gain knowledge that may be applicable to part or all of a particular virus family. These representative viruses are considered prototype pathogens. For example, within the Arenaviridae family, Lassa, Junin, or other viruses could be selected as a prototypic pathogen(s). The ideal arenavirus prototype pathogen(s) would not only be a virus with a risk of causing an outbreak, but most importantly, would be one that shares functional and structural properties with viruses across the Arenaviridae family. Thus, increasing fundamental knowledge and developing MCMs for the prototype virus(es) not only provides ready potential solutions for these viruses, but also the framework for a rapid research and product development response to other viruses within that family should an outbreak occur.
Excerpt from NIAID Pandemic Preparedness Plan
The goals for the NIAID Pandemic Preparedness Plan are to:
- Systematically characterize pathogens of concern and increase research and surveillance to
identify threats before they emerge - Shorten timelines between pathogen emergence or outbreak onset and authorization/approval
of candidate diagnostics and medical countermeasures, such as therapeutics and vaccines - Bridge or eliminate existing gaps in research, infrastructure, and technology and expand pre-
clinical and clinical testing capacity
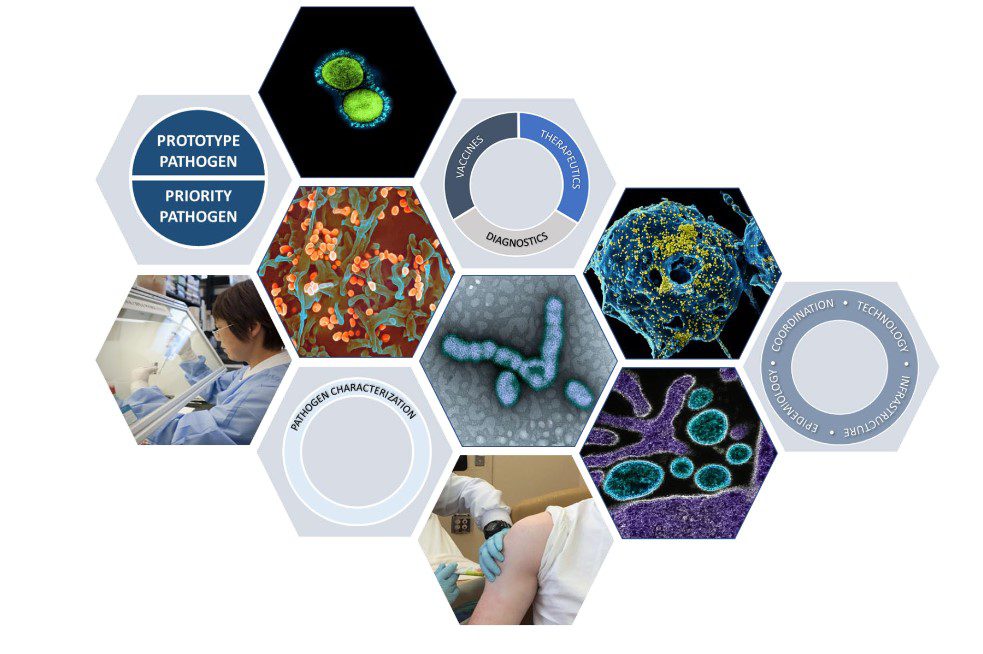
NIAID will apply this knowledge to conduct translational and clinical research to develop diagnostics; therapeutics, including antivirals, monoclonal antibodies, and broad-spectrum approaches; and vaccines. These efforts are designed to shorten timelines between pathogen emergence and authorization/approval of candidate products.
Developing animal models that recapitulate human disease is a vital step toward understanding disease pathogenesis and mechanisms of immune protection as well as the assessment of MCM efficacy. Small animal models will be prioritized to enable rapid, scalable studies particularly valuable for screening countermeasure candidates for immunogenicity, drug candidate pharmacokinetics/pharmacodynamics, vaccine and therapeutic efficacy and safety. In parallel, development and characterization of large animal models, including non-human primates that closely recapitulate human disease, is pivotal to advance promising candidates toward clinical evaluation. NIAID will support the development of essential small and large animal models to better understand viral biology and to assist in vaccine and therapeutic development. NIAID also will ensure that well characterized animal models that recapitulate human disease are made available to the scientific community for evaluating priority MCMs.
Excerpt from NIAID Pandemic Preparedness Plan
The institute’s comprehensive preparedness efforts will also include novel epidemiology and pathogen discovery programs, pre-clinical and clinical infrastructure capacity, technology enhancements to hasten therapeutic and vaccine development, and a robust and coordinated communication structure, according to the plan. NIAID will continue to collaborate with partners in the U.S. and foreign governments, the biopharmaceutical industry, and international organizations on its preparedness efforts.
The new plan was informed by a November 2021 workshop NIAID hosted to facilitate discussions with the scientific community about the development of a pandemic preparedness strategy and prioritizing prototype pathogens within viral families of concern. Moving forward, NIAID will continue to engage the scientific community and U.S. and global partners to ensure preparedness planning efforts are collaborative, integrated, and aligned with current scientific research.
Source: National Institutes of Allergy and Infectious Diseases


